Researchers at Columbia University’s Mortimer B. Zuckerman Mind Brain Behavior Institute and Columbia University Medical Center (CUMC) have identified a cellular circuit that helps the mouse brain to remember which environments are safe, and which are harmful. Their study also reveals what can happen when that circuitry is disrupted — and may offer new insight into the treatment of conditions such as posttraumatic stress, panic and anxiety disorders.
The researchers published their findings today in the journal Science.
Learning and memory are among the brain’s most fundamental tools for survival. Accurate encoding of ‘contextual’ memories — those associated with particular experiences — enables us to exhibit the appropriate fear responses and, importantly, avoid dangerous situations. Of equal importance is the brain’s ability to discriminate between an environment that it has previously learned to be dangerous and one that is safe.
Without this delay, fearful memories lack specificity and accuracy, preventing the brain from appropriately distinguishing danger from safety.
Earlier research demonstrated that contextual memories are formed and stored in two interconnected brain regions: the hippocampus and the entorhinal cortex, which are involved in memory and navigation. These two regions are linked via a complex network of brain cells, or neurons. Although scientists have been able to determine how most of this network operates, one connection has remained puzzling.
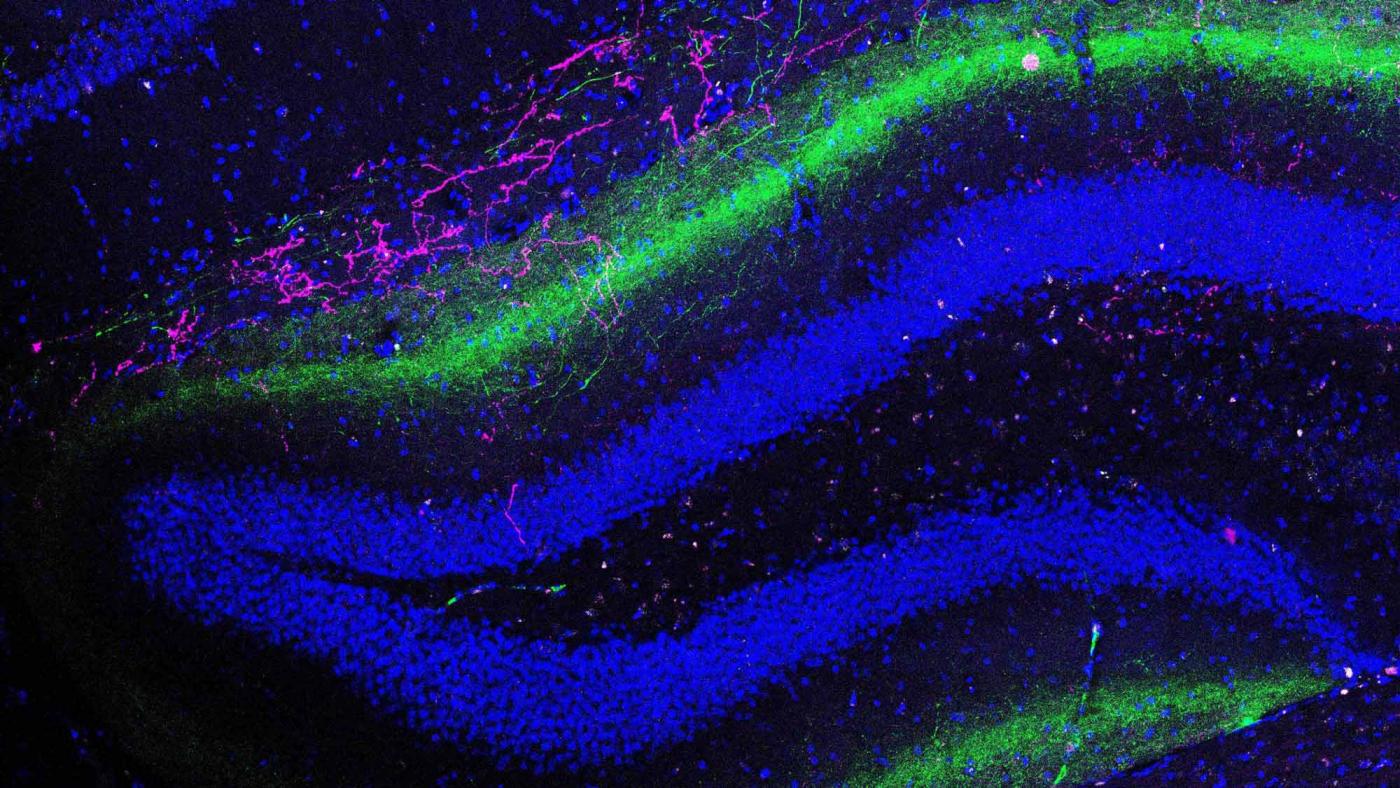
“Neurons in the entorhinal cortex wind their way into the hippocampus via two distinct routes, or pathways,” explained Jayeeta Basu, PhD, an assistant professor in neuroscience and physiology at the NYU Neuroscience Institute. Dr. Basu was this study’s first author and is a former postdoctoral fellow in the laboratory of Steven Siegelbaum, PhD, at CUMC. “It is thought that contextual memories are formed when these two pathways become activated as part of a carefully timed sequence. But a few years ago, scientists discovered a third pathway that linked the two regions whose purpose was unknown.”
About 80 percent of neurons in the brain are excitatory — meaning that they carry communications long distances across brain regions, while the other 20 percent are inhibitory. These inhibitory neurons act locally to slow or halt the excitatory activity — much like tapping the brake pedal after a period of acceleration. What was so unusual about the neurons in this third pathway was that they acted across a relatively long distance, but were also inhibitory. So scientists called them long-range inhibitory projections, or LRIPs.
The purpose of this study was to investigate the role these LRIPs may play in learning and memory. First, the researchers temporarily silenced them in the mouse brains. Then, the mice were placed in a room where they were given a brief but aversive footshock. When returned to the same room 24 hours later, the mice remembered the shock and exhibited a fear response, indicating that LRIPs were not required for the formation of fearful memories.
But when placed in a completely different room, these mice again exhibited fear, suggesting they were generalizing their initial fear in a different context. This is in stark contrast to what was observed in normal, healthy mice, which only exhibited a fear response in the room where they had been shocked—and thus revealing their ability to distinguish between dangerous and neutral environments.
Additional imaging experiments and electrical recordings from normal, healthy mouse brains revealed the precise role of LRIPs in astounding detail. Normally a stimulus — such as a sound, light or small footshock — activates the LRIPs, which send an inhibitory signal from the entorhinal cortex into the hippocampus. Upon arrival, the LRIP signal actually inhibits another set of inhibitory neurons in the hippocampus. This then frees up neurons in the hippocampus to switch on and, ultimately, generate a memory.
This seemingly confusing series of signal relays is actually part of a sophisticated gating mechanism, as evidenced by a short, 20-millisecond delay between when the LRIPs are initially activated, and when their inhibitory signals arrive in the hippocampus.
“We found that this brief delay actually enables the electrical signals to flow into the hippocampus in an elegant, precisely timed sequence, which is ultimately what allows the memory to form and be stored with the appropriate specificity so that it can be recalled accurately,” said Dr. Siegelbaum, who is a principal investigator at the Zuckerman Institute, chair of the Department of Neuroscience at CUMC and senior author of the paper. “Without this delay, fearful memories lack specificity and accuracy, preventing the brain from appropriately distinguishing danger from safety.”
“The implications of these findings for the human brain, while preliminary, are intriguing,” said Attila Losonczy, MD, PhD, an assistant professor of neuroscience at CUMC, a principal investigator at the Zuckerman Institute, and a co-author of this study. “The study suggests that any alterations in these pathways activity — particularly a disruption of the timed delay — may contribute to pathological forms of fear response, such as posttraumatic stress, anxiety, or panic disorders.”
###
This paper is titled “Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition.” Jeffrey Zaremba, Stephanie Cheung, MD, Frederick Hitti, MD, PhD, and Boris Zemelmen, PhD, also participated in this research.
This research was supported by the Brain and Behavior Research Foundation, the National Institute of Mental Health (MH100510, MH100631), the Searle Scholars Program, the Human Frontier Science Program, the McKnight Foundation, the National Institute of Neurological Disorders and Stroke (NS036658) and the Howard Hughes Medical Institute.
The authors report no financial or other conflicts of interest.