NEW YORK – In 2013 neuroscientist Franck Polleux got a call from his colleague Dietmar Schmucker in Belgium, who had noticed something strange while studying the development of brain cells (neurons, specifically) in flies. Switching off a single gene, which until then had been associated mostly with hypertension and kidney function, led to serious defects in an insect’s neurons.
Now, after 8 years of collaborative experiments, Polleux and Schmuker’s teams have discovered just how critically important this Wnk gene (and a related pair of Wnk genes in mice) is for neurons’ development and maintenance in the adult brain. As reported on August 11 in Neuron, Wnk genes (pronounced “wink”) help young neurons grow the long branching projections (axons) that carry information in the form of electrical impulses around the brain and body. Surprisingly, the scientists also discovered that Wnk keeps fully formed axons intact in the adult brain.
“We found that the health of neurons depends on Wnk, which must always be active to prevent axons from spontaneously degenerating,” said Dr. Polleux, PhD, a principal investigator at Columbia’s Zuckerman Institute and co-corresponding author on the new paper. “This gene is among the first genes identified to play such a striking role.”
Exploring Wnk’s power over the life and death of axons could ultimately further efforts to treat injured and diseased brain cells, the researchers hope.
“Nature is playing a dangerous game by giving this gene so much sway,” said Dr. Polleux. “The more we understand the rules of this game, the greater the potential for developing new ways to repair damage to the brain.”
Fly Screens
The new research began in the lab of Professor Dietmar Schmucker, PhD, who was at KU Leuven in Belgium and recently moved to the University of Bonn’s Life & Medical Sciences Institute in Germany. His team studies a specific nerve cell that connects a fly’s brain to a skin bristle the insect uses to touch and taste its surroundings. The cell has a long axon with treelike branches at the end, arranged in a well-defined shape.
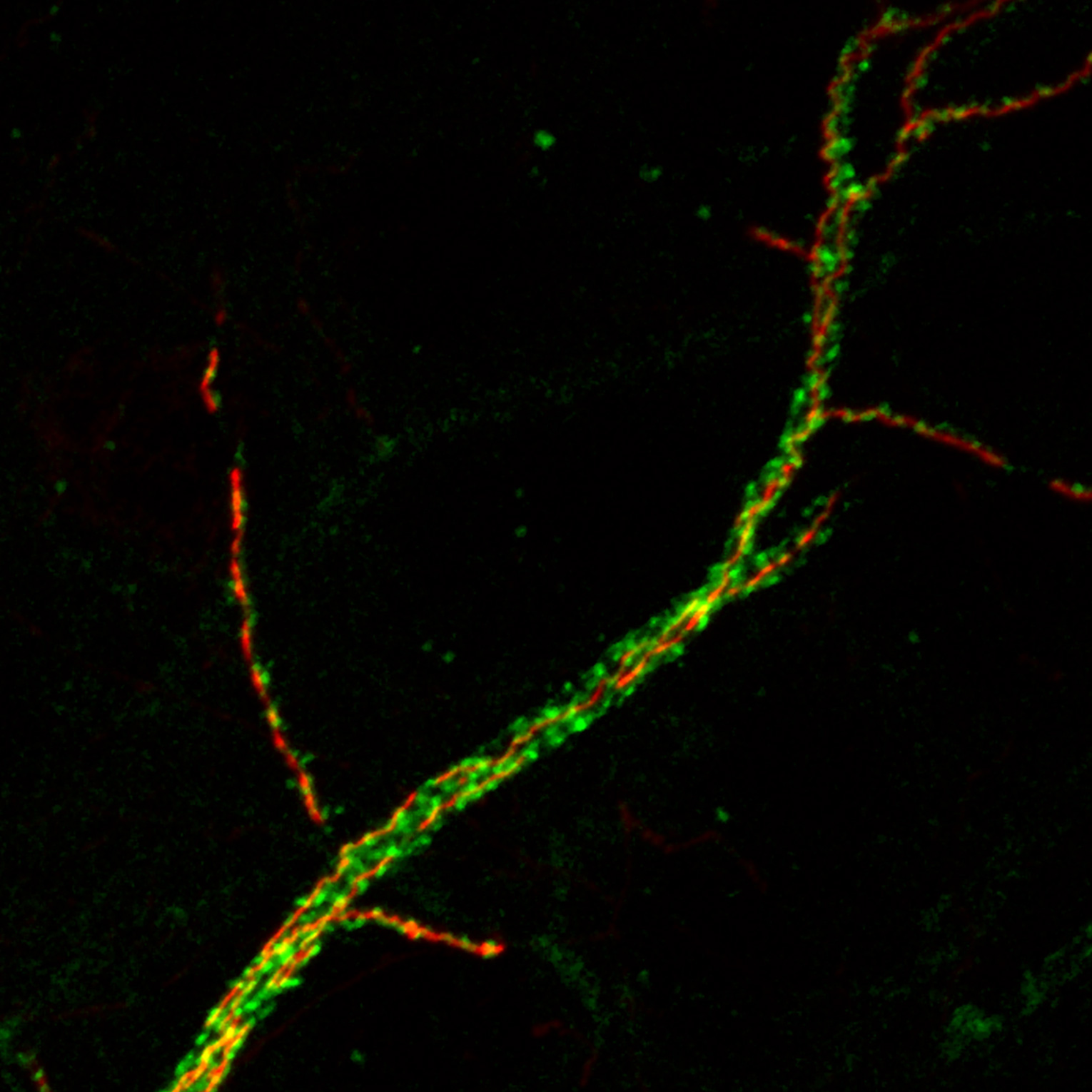
The scientists wanted to know what genes influence that shape. So they screened hundreds of genes, switching them off one by one in hundreds of flies. After a year of work, the researchers found that disabling one gene in particular, Drosophila Wnk, prevented nerve cells in young flies from developing proper axons; it also caused the axons of cells in adult flies to disintegrate over the course of days.
“We saw really dramatic degeneration happen in a relatively short period of time,” said Dr. Schmucker, co-corresponding author on the new paper. “The long axon of the nerve cell fragmented and then completely disappeared.”
Wink Wink
Building on this work, Dr Julien Courchet, a former postdoctoral fellow in Dr. Polleux lab, investigated a pair of similar genes in the brains of mice: Wnk1 and Wnk2. His lab turned off these genes in hundreds of select neurons, using a technique called RNA interference.
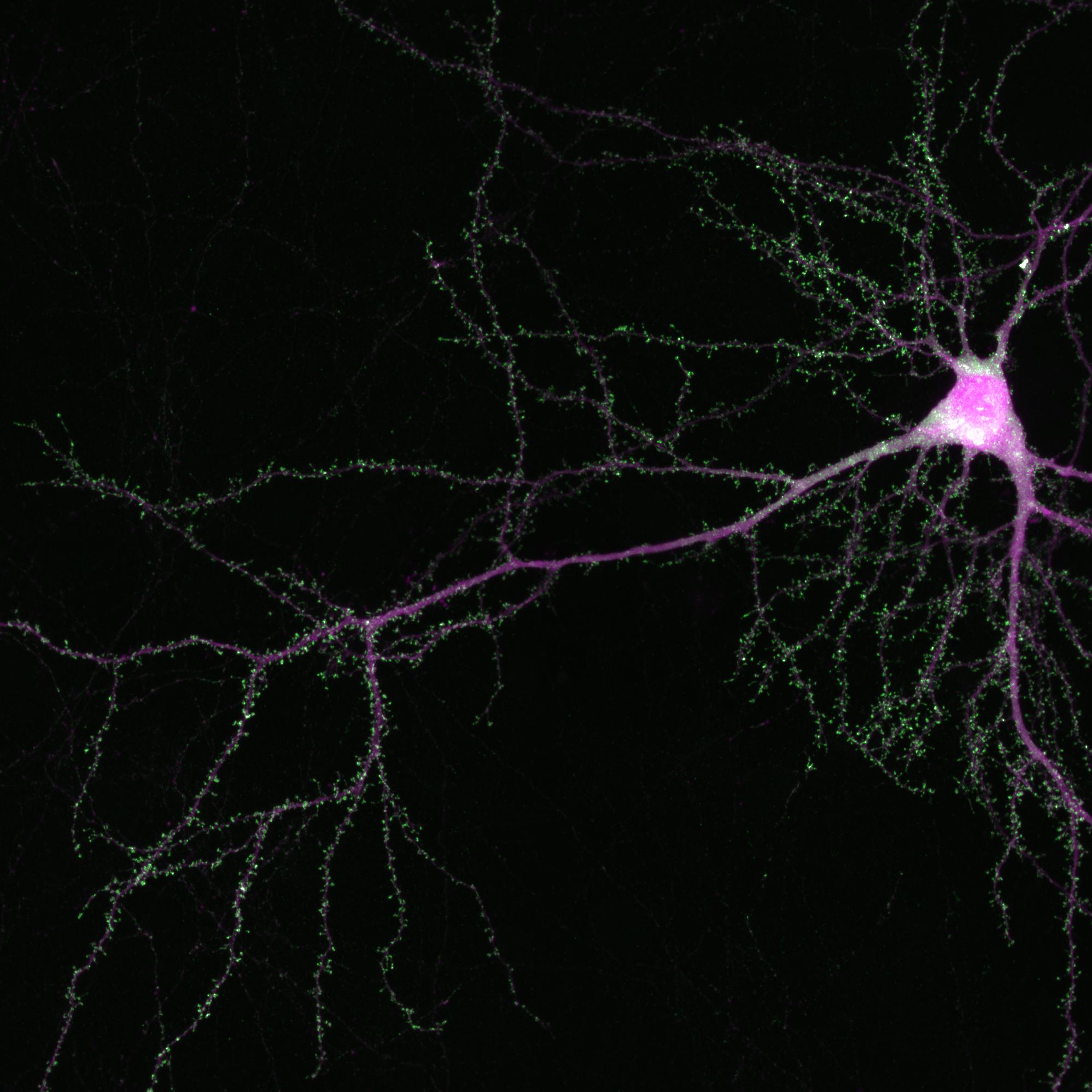
Disrupting the Wnk gene pair in the developing brains of infant mice prevented neurons from forming and consolidating axon branches, just as in the original fly experiments. It also caused fully formed axons in the brains of adult mice to suddenly degenerate, just as in the original fly experiments. The same thing happened to axons of isolated cells grown in a dish.
“We saw a very striking difference upon downregulation of Wnk1 or Wnk2,” said Dr. Polleux, who is also a professor of neuroscience at Columbia’s Zuckerman Mind Brain Behavior Institute. “Axons, which usually possess these long, beautiful branches, turned into little stubs and seem to literally disintegrate.”
The similar findings obtained in flies and mice suggest that Wnk plays an evolutionary conserved role for brain health throughout the animal kingdom, including humans.
“The biggest surprise in these experiments, which still remains somewhat mysterious, was the connection between development and maintenance,” said Dr. Schmucker. “We found growth and selection mechanisms in the embryonic brain that are not shut off in the adult brain, and we think these mechanisms could be affected by disease.”
Our surprising discovery will trigger a lot of interest among other scientists
How Axons Get Axed
Scientists know that a neuron sometimes destroys its own axon when damaged. Nick a nerve, and the molecular skeleton keeping its axon intact will break down. For nerves running through arms and legs, this process can be the first step toward regeneration. For neurons in the brain, this cellular reset button has been linked to neurological disorders such as ALS, Parkinson’s and Alzheimer’s disease.
Previous studies of how neurons induce destruction of their axon in response to injury have identified some of the molecules responsible in flies: specifically, dSarm and Axed. The Polleux lab and their colleagues found evidence that these molecular executioners are kept in check by Wnk. The scientists also discovered that Wnk positively interacts with Nmnat to regulate axon branching and prevent axon degeneration. Interestingly, Nmnat has recently been found to protect axons from degenerating in neurodegenerative diseases, such as Parkinson’s and certain forms of dementia.
Further work will be needed to tease out the details of how all these players work together. But given how little was known about the role of Wnk before the new experiments, Drs. Polleux and Schmucker think their work offers a new pathway for improving brain repair and for protecting neurons from degeneration.
“Our surprising discovery will trigger a lot of interest among other scientists,” said Dr. Polleux. “We’ve uncovered a completely novel function for Wnk in neuronal development and degeneration, which is going to open up a lot of new investigations.”
###
This paper is titled “Axon morphogenesis and maintenance require an evolutionary conserved safeguard function of Wnk kinases antagonizing Sarm and Axed.” Authors on the paper are Azadeh Izadifar, Julien Courchet, Daniel M. Virga, Tine Verreet, Stevie Hamilton, Derya Ayaz, Anke Misbaer, Sofie Vandenbogaerde, Laloe Monteiro, Milan Petrovic, Sonja Sachse, Bing Yan, Maria-Luise Erfurth, Dan Dascenco, Yoshiaki Kise, Jiekun Yan, Gabriela Edwards-Faret, Tommy Lewis, Franck Polleux and Dietmar Schmucker.
This work was supported in Dr. Polleux’s lab by a grant from NINDS and from the Ludwig Family Foundation.
The authors declare no financial or competing interests.