From an eight-armed octopus to a caterpillar with 12 eyes, animals have evolved an incredible array of shapes and sizes. This diversity of body plans starts with blueprints written into DNA, genes that guide the development of every creature on the planet.
Richard Mann, PhD, has been studying a group of genes known as the Hox family, which help embryos grow body parts in the proper number and arrangement. By deciphering how fruit flies develop their wings, legs and other appendages, he hopes to shed light on how the human nervous system grows inside the body.
Although scientists have spent decades investigating how Hox genes work, much remains unknown about how they actually operate. Dr. Mann, a principal investigator at Columbia’s Zuckerman Institute and the Higgins Professor of Biochemistry and Molecular Biophysics (in Systems Biology) at Columbia University's Vagelos College of Physicians and Surgeons, investigates the way in which proteins made by Hox genes bind to DNA. He has co-authored a new paper, appearing August 5 in Current Biology, that reveals new insights on how Hox genes switch other genes on and off.
We spoke with Mann and Ryan Loker, the first author of the new study and a PhD student in Mann's lab, about what their latest research has revealed, what it could mean for our understanding of the architecture of bodies in all animals and how it might one day help treat neurodegenerative disorders impacting mobility.
So the Hox genes are the master architects of the body?
Ryan Loker: Hox genes are most well known for their role in telling a cell in a developing embryo its position: how close it is to the head versus the tail. They perform this function in all animals, and are potentially even more ancient than animals.
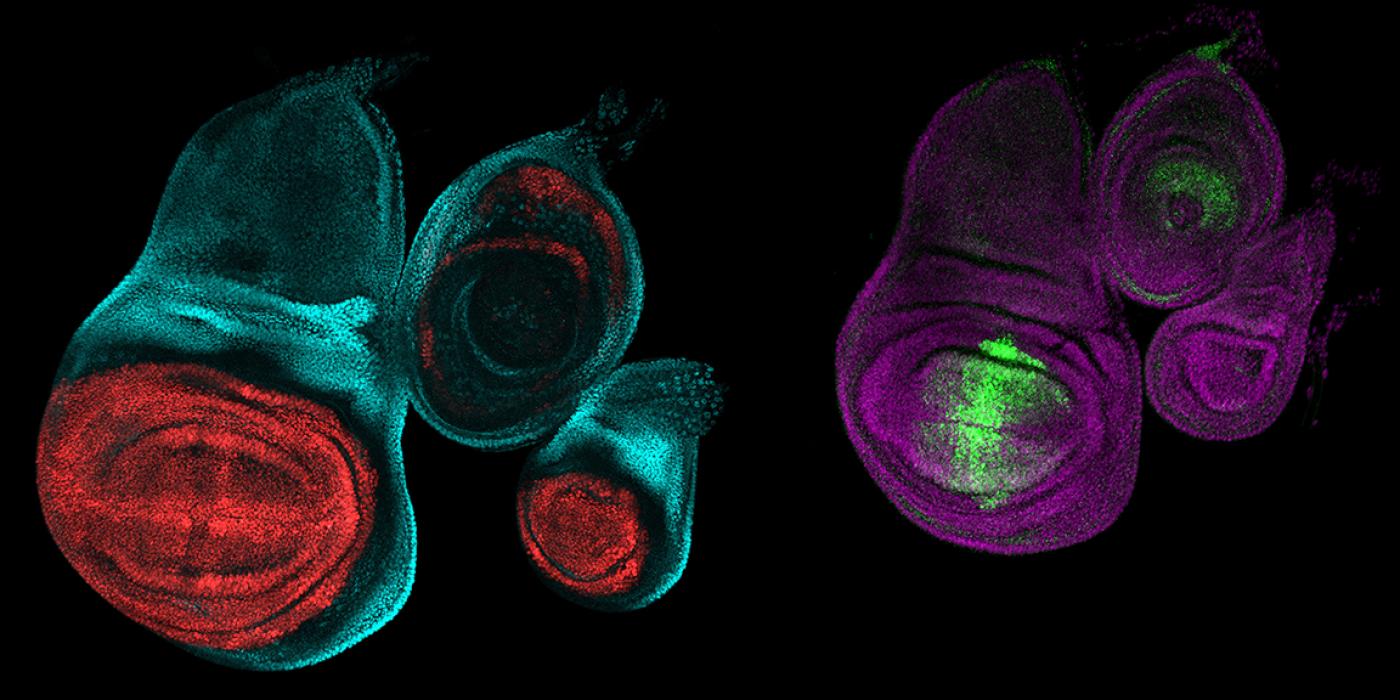
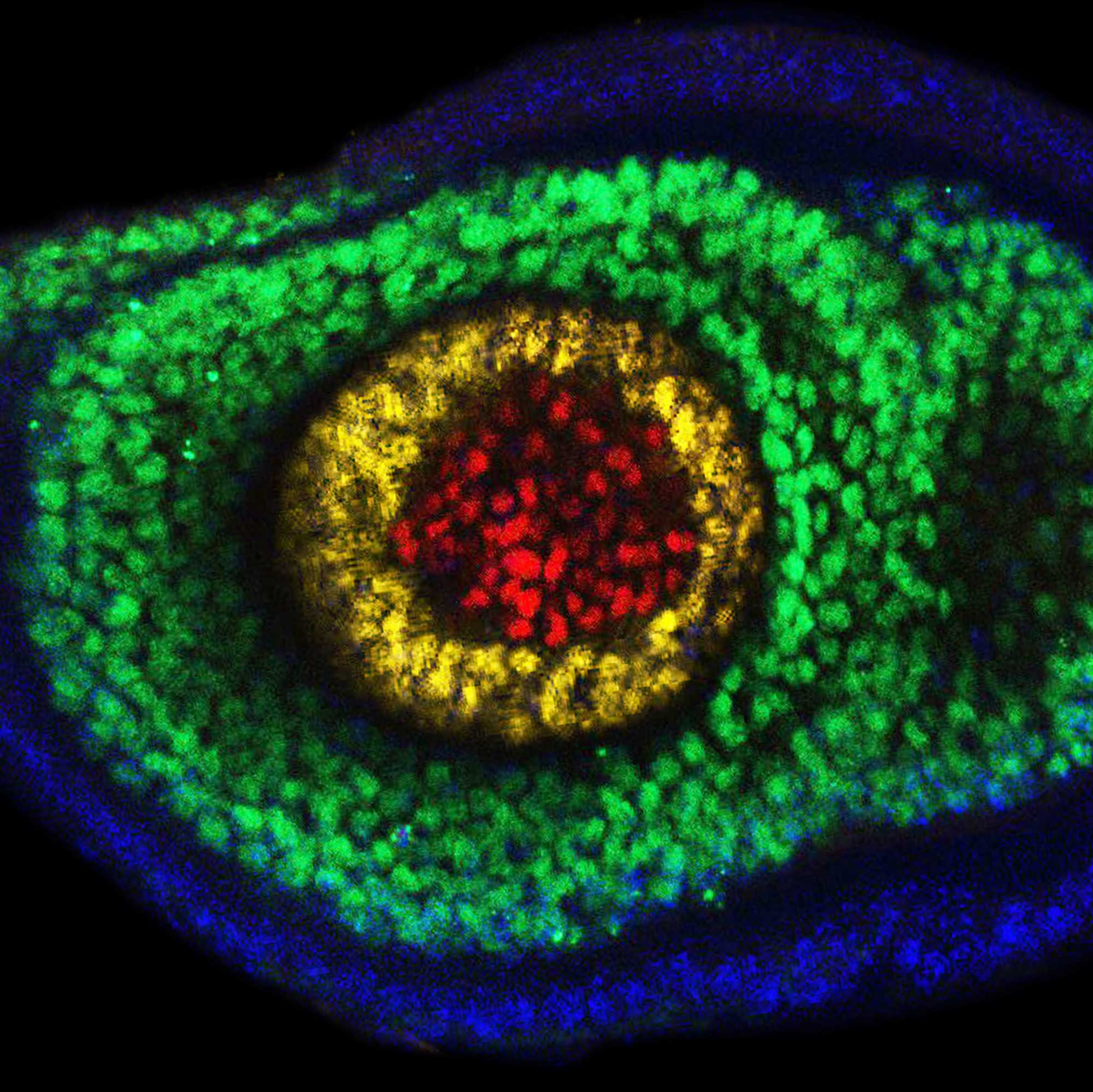
Richard Mann: One classic illustration of what Hox genes do comes from the fruit fly Drosophila melanogaster. This middle part of a fly’s body, the thorax, has three segments. The second segment is where its two large wings sprout. The third segment usually sports a pair of halteres: small, bulbous hindwings that balance the fly during flight, like a gyroscope, helping an insect fly in a straight line. If you remove the Hox gene Ultrabithorax from the third segment, something dramatic happens. As first revealed by the fruit fly geneticist E.B. Lewis, the third thoracic segment transforms into a nearly identical copy of the second thoracic segment. Instead of forming halteres, the fly sprouts an extra pair of large wings, giving the insect four wings in total.

Picture of a wild type fly (with only one pair of wings) next to the four-winged bithorax mutant.
How do Hox genes work?
Mann: They produce transcription factors, proteins that bind to DNA and turn genes on and off. This is what Hox genes do in all animals, whether you're a mouse or a human or a fly.
Loker: Despite this shared mechanism, a Hox gene might produce a really wide variety of outcomes in different animals. As animals evolve, Hox proteins change what DNA sites they bind to. Different genes get turned on or off, producing a different array of appendage types in different species over time. Changes in Hox proteins and the genes they control underlie how a four-legged lizard can evolve into a limbless snake, for instance.
Why do you study Hox genes in flies?
Mann: Scientists have developed a lot of amazing tools for studying and manipulating genes in flies. It’s much easier to investigate these genes in flies than in humans and other vertebrates.
Loker: And much less expensive, too.
How might studying what Hox genes do in fruit flies shed light on the human condition?
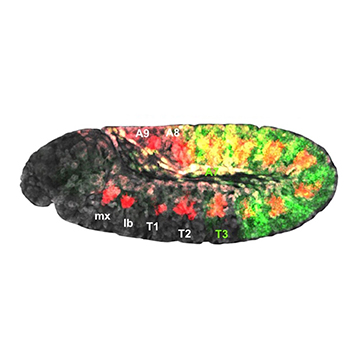
Loker: Just as Hox genes pattern the body plan of a fruit fly, they also control the layout of the human spinal cord. As a human fetus grows, a particular Hox gene might switch on in one part of its developing spinal cord to give rise to motor neurons that control the leg. In another part, another Hox gene might produce neurons that control muscles in the abdomen.
Mann: If we understand these processes, we may be able to create new ways to repair the nervous system. Hynek Wichterle's lab at Columbia University is using Hox genes to help transform stem cells from mammals into different types motor neurons. The long-term goal for their work is to generate motor neurons in a dish that can be used for cell therapy for people who have motor neuron degeneration diseases.
If we understand these processes, we may be able to create new ways to repair the nervous system.
So what did you discover about Hox genes in your latest work?
Loker: We looked at how that Hox gene Ultrabithorax determines whether a fly grows a pair of large wings or a pair of tiny halteres on a thorax segment. We found that this gene is associated with changes to chromatin, which is how DNA is packaged in the cell nucleus. By changing chromatin, the transcription factors produced by Ultrabithorax can control how well other transcription factors can access their binding sites in the DNA.
Mann: Ultrabithorax changes what other transcription factors can or cannot do in a tissue. And, according to Ryan's work, to make a haltere instead of a wing Ultrabithorax both increases and decreases how accessible the chromatin is to other transcription factors.

Side view of a four-winged bithorax fly mutant.
So the fruit fly doesn't require two different Hox genes to do two different things for the wing and the haltere? Instead of needing, say, two different utensils, like a fork and spoon, Ultrabithorax can operate like a spork?
Mann: That's the beauty of the system. Ultrabithorax can do both things. It helps answer age-old questions about how Hox genes go about regulating large numbers of genes to make dramatic changes in appendage morphology.
How does Ultrabithorax know how to do something differently in the wing versus the haltere?
Loker: Over time, in evolution, thousands of different parts of the genome acquired sites to which Ultrabithorax can bind. It only targets those specific binding sites.
Mann: It's important to emphasize that Hox proteins are not working by themselves. When one binds to a particular sequence of DNA, there are probably 20 other transcription factors, plus or minus, that can bind to that site and influence the timing of when a gene is turned on or off or exactly which subset of cells it will act in within a tissue.
What directions are you going from here with your work?
Loker: There are a lot of questions that are still open within the field. Now that we know where it binds, we can begin to understand which genes Hox genes target to change morphologies. How do the specific genes they target in one part of the haltere differ in another part of the haltere? What other transcription factors does a Hox gene like Ultrabithorax work with in specific cell types to turn genes on and off in a specific way?
Mann: We want to learn more about exactly how Ultrabithorax opens and closes chromatin. It must recruit other proteins that change chromatin accessibility, but we don’t know the details of this yet.
And this research with Hox genes in fruit flies will likely apply to humans as well?
Mann: That's always been our assumption. Every time this assumption has been tested, it's been confirmed.
Loker: We presume that a lot of what we uncover in the fly will be relevant to spinal cord development and also human brain development.
Now that you've made these discoveries in fruit flies, do you expect others to hunt for similar findings in humans and elsewhere?
Mann: Definitely. I certainly hope that this work will stimulate other people to think about these genes differently.
###
To continue exploring this research, see the new paper: "Cell type-specific Hox regulatory strategies orchestrate tissue identity." Published August 5, 2021, in Current Biology, by Ryan Loker, Jordyn Sanner and Richard Mann.