To say that evolution is complex would be an understatement. But every once in a while, it can also be elegant in its simplicity. In a study published this June in Neuron, Zuckerman Institute Principal Investigator Franck Polleux, PhD, and colleagues described a stunning example of human evolution — one that may have guided the development of the human brain.
We spoke with Dr. Polleux, the paper’s co-senior author, about his discovery.
What propelled you to study human evolution?
I have long been interested in understanding the genetic changes that drove the evolution of the human brain. It’s one of the biggest questions in biology: How did our brains develop the ability to create a piece of music or learn a language? Ultimately, the answers to these questions are encoded in our DNA — we just have to know where to find them.
Within the last decade, researchers began to notice peculiarities in the human genome that we found intriguing. We called them human-specific gene duplications.
What is a gene duplication?
A gene duplication occurs when a single piece of DNA is copied and then inserted elsewhere in the genome. Gene duplications occur in all living organisms. But scientists have recently identified more than 30 gene duplications that are unique to humans. Early on, we speculated that because these particular duplications are found only in the human genome, they might be tied to some of our uniquely human traits — both of brain development and function — that ultimately allow for the emergence of cognitive abilities such creativity, language and problem-solving.
In 2006, we started studying the duplication of the gene SRGAP2. The genomes of virtually all mammals today contain one copy of SRGAP2, called SRGAP2A. But in addition to SRGAP2A, humans also have a second, slightly different version of this gene, which we named SRGAP2C.
What have you uncovered about SRGAP2C?
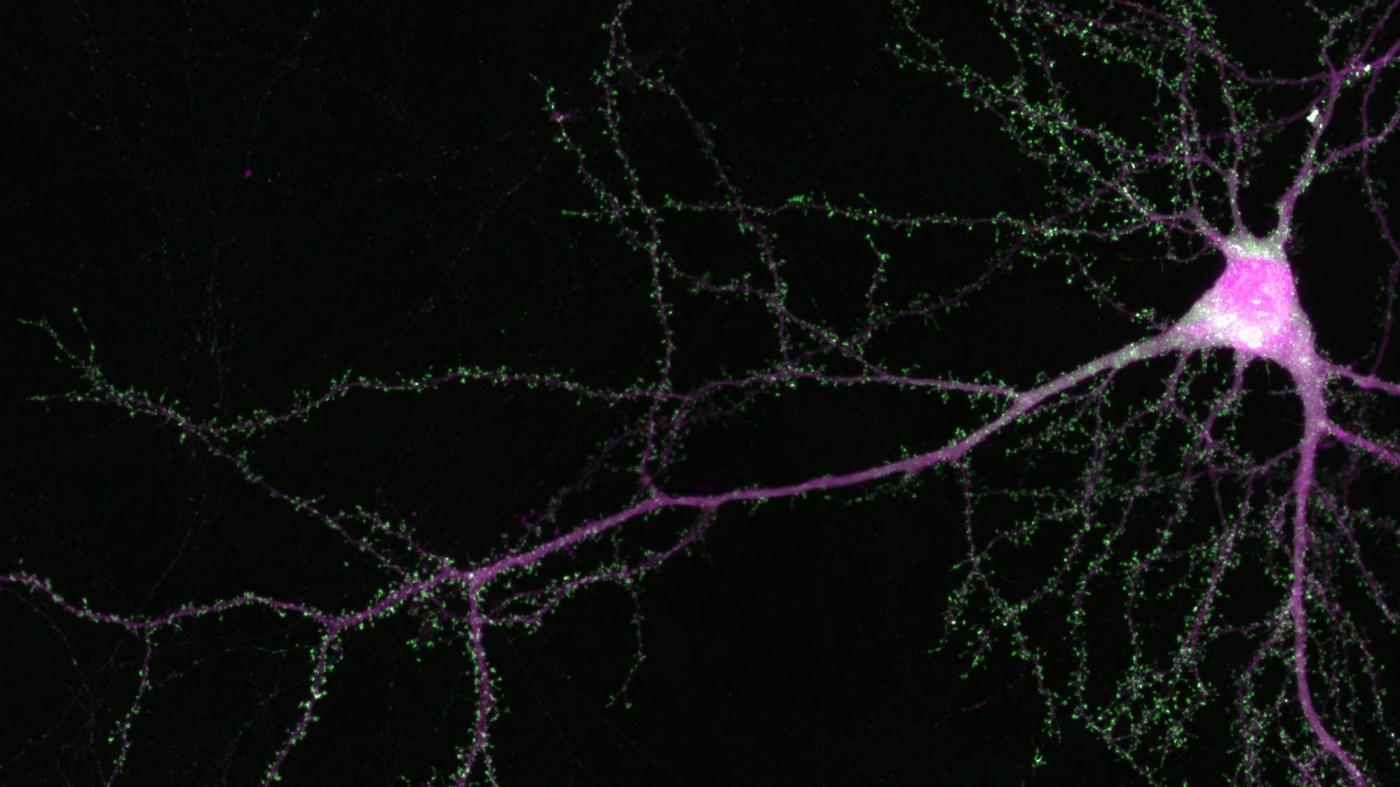
My lab and others found that SRGAP2A — which is present in all mammals, including humans — plays two major roles in the brain and more specifically it is critical for synapse development. Synapses are connections between neurons that allow for information (in the form of electricity) to pass between cells. The more synapses a neuron has, the more information it can process. First, SRGAP2A speeds up the maturation of synapses so that they reach adult-like function faster. Second, SRGAP2A limits the number of synapses per neuron. Taken together, SRGAP2A’s two major functions essentially create an upper limit to how much information the brain can compute.
But in humans, the emergence of SRGAP2C somehow erases that upper limit, allowing for the formation of more synapses. At the same time, SRGAP2C also slows down the growth process, so that these synapses take longer to mature. This one-two punch results in neurons that have stronger and denser connections to their neighbors, and are therefore better able to transmit and store information. This feature, many have argued, may very well underlie the evolution of our species’ unmatched brainpower.
This initial discovery was the core of our 2012 paper in Cell, but many questions remained. At the time, we had no idea how SRGAP2A and SRGAP2C were able to accomplish this.
What made SRGAP2C’s existence in the human brain so puzzling?
Earlier I mentioned a neuron’s connections, or synapses, which regulate the flow of information in the brain. There are actually two kinds of synapses: excitatory synapses, which enhance electrical signals, and inhibitory synapses, which constrain them. In general, about 90 percent of synapses on a neuron are excitatory, while 10 percent are inhibitory. Together, these two types of synapses work in tandem to ensure normal brain function — like the gas pedal and the brake — to control proper flow of information.
What’s fascinating is that if you peer inside the brains of nearly any animal on the planet, you’ll see the same 90-to-10 percent ratio — an indication that it is critical for normal brain development and function. If you tweak this ratio even slightly — to, say, 95-to-5 percent — neurons become overly excited, which can have all sorts of negative effects, such as seizures. In fact, this hyper-excitability has been observed in epilepsy, as well as many other neurodevelopmental disorders such as autism.
However, the importance of this ratio presented a mystery. We knew that SRGAP2C boosted the overall density of synapses in the human brain, but we couldn’t figure out how it was able to boost the density of both types of synapses without altering that 90:10 ratio. Answering that question was the focus of this most recent study.
What did you find out?
When Dr. Cécile Charrier (a postdoc in my lab and the paper’s co-senior author), and I were initially thinking about what could be happening, we envisioned two scenarios. Either SRGAP2C had a partner, so that while it boosted excitatory synapses another, yet-to-be-discovered human-specific gene did the same to inhibitory synapses (thereby keeping the 90:10 ratio constant). Or, alternatively, SRGAP2C was somehow able to affect the growth of both kinds of synapses simultaneously – in one fell swoop.
In our experiments, we found the latter to be true — in a startlingly simple way.
As I mentioned, SRGAP2A puts tight restrictions on the way synapses form and mature. Further investigation found that it does so by controlling many moving parts. Rather than try and alter each moving part individually, SRGAP2C goes after the source: SRGAP2A. Essentially, it blocks SRGAP2A from doing what it would have normally done. This results in a boost in neuronal strength and density — and keeps that critical 90:10 ratio in check.
Now that you’ve uncovered SRGAP2C’s role, what are your next steps?
We now understand how SRGAP2C boosts both excitatory and inhibitory synapses. We know what molecules are involved in the mechanism. But we still don’t know how this boost in synaptic strength and density translates to brain function and behavior. Right now, we’re very interested in whether we can see a difference in cognition, memory formation or other types of advanced brain functions in animals that have been genetically modified to express SRGAP2C, compared to those that have not.
In collaboration with fellow Zuckerman Institute investigator Attila Losonczy, MD, PhD, my lab is now beginning to test whether mice genetically engineered to express SRGAP2C show enhanced brain function and maybe cognitive abilities.
What excites you most about these results?
We are amazed at the simplicity of this mechanism — that a single genetic change (in this case, the emergence of SRGAP2C) can have such a huge effect on brain development.
The second thing that intrigues me is the timing of when SRGAP2C first makes an appearance in the evolutionary timeline. The initial estimate suggested that SRGAP2C first appeared about 3 million years ago. We are trying to refine this date, but it would be intriguing if SRGAP2C preceded — and maybe even contributed to — the emergence of our genus, Homo, and along with it, not only an increase in brain size but more importantly improved brain function.
Could the two be related? That’s something we’re still investigating, but the possibilities are exciting.
###
This paper is entitled “SRGAP2 and its human-specific paralog co-regulate the development of excitatory and inhibitory synapses.” Additional contributors include Matteo Fossati, Rocco Pizzarelli, Ewoud R. Schmidt, Justine V. Kupferman, David Stroebel and Cécile Charrier. This work was supported by a grant from the National Institutes of Health, the Kavli Foundation for Brain Sciences and an award to Dr. Polleux from the Fondation Roger de Spoelberch.