More than 30 years ago, scientists discovered that neurological illnesses such as mad cow and Creutzfeldt-Jakob diseases are caused by misfolded proteins called prions. But in recent years, Nobel laureate Eric Kandel, MD, demonstrated in mice that some prions are beneficial and serve important biological functions in the brain and body. And today, new Columbia research from Dr. Kandel and his colleague Joseph Rayman, PhD, describes how one such prion-like protein, encoded by a gene called TIA1, helps the brain keep fearful memories in check. Without this gene, female mice exhibit the tell-tale signs of post-traumatic stress disorder, or PTSD.
The results of this study, published today in Cell Reports, point to TIA1 as a new target in the fight against PTSD, which is about twice as common in women as in men.
“Deciphering the interplay between biology and environment that gives rise to all psychiatric disorders, including PTSD, has proven incredibly difficult,” said Dr. Kandel, who is University Professor and codirector of Columbia’s Mortimer B. Zuckerman Mind Brain Behavior Institute, as well as Kavli Professor of Brain Science at Columbia. “Today’s discovery about TIA1 has uncovered one such key component to that interplay. Our research offers a promising way forward for developing treatments that mitigate the underlying causes of PTSD and other related psychiatric illnesses.”
Advances in computational and theoretical modeling could soon allow us look at a person’s particular genetic composition and identify his or her risk for developing a psychiatric disorder, such as PTSD.
Prions were originally described in the context of neurological conditions called transmissible spongiform encephalopathies, which include mad cow disease in cattle and Creutzfeldt-Jakob disease in people. In these diseases, the misfolded prions clump together inside brain cells called neurons, forming large aggregates that cause neurons to degrade and die. Upon neuronal death, the prions are released and infect neighboring cells, much like a virus. Over time, this process leads to devastating and sometimes deadly neurological symptoms.
But in 2003, Dr. Kandel and his team discovered that some prions are not dangerous, but functional, and can play important biological roles. For example, in 2015, Dr. Kandel and his team discovered that the functional prion CPEB3 helps the brain maintain long-term memories. TIA1 is another such functional prion. It helps neurons cope with cellular stress.
“When neurons undergo stress, such as in response to a viral infection, the TIA1 protein sequesters non-essential biomolecules inside the cell,” said Dr. Rayman, who is an associate research scientist in the Kandel lab at Columbia’s Zuckerman Institute and the paper’s first author. “This allows the cell to focus all its efforts on fighting the stress.”
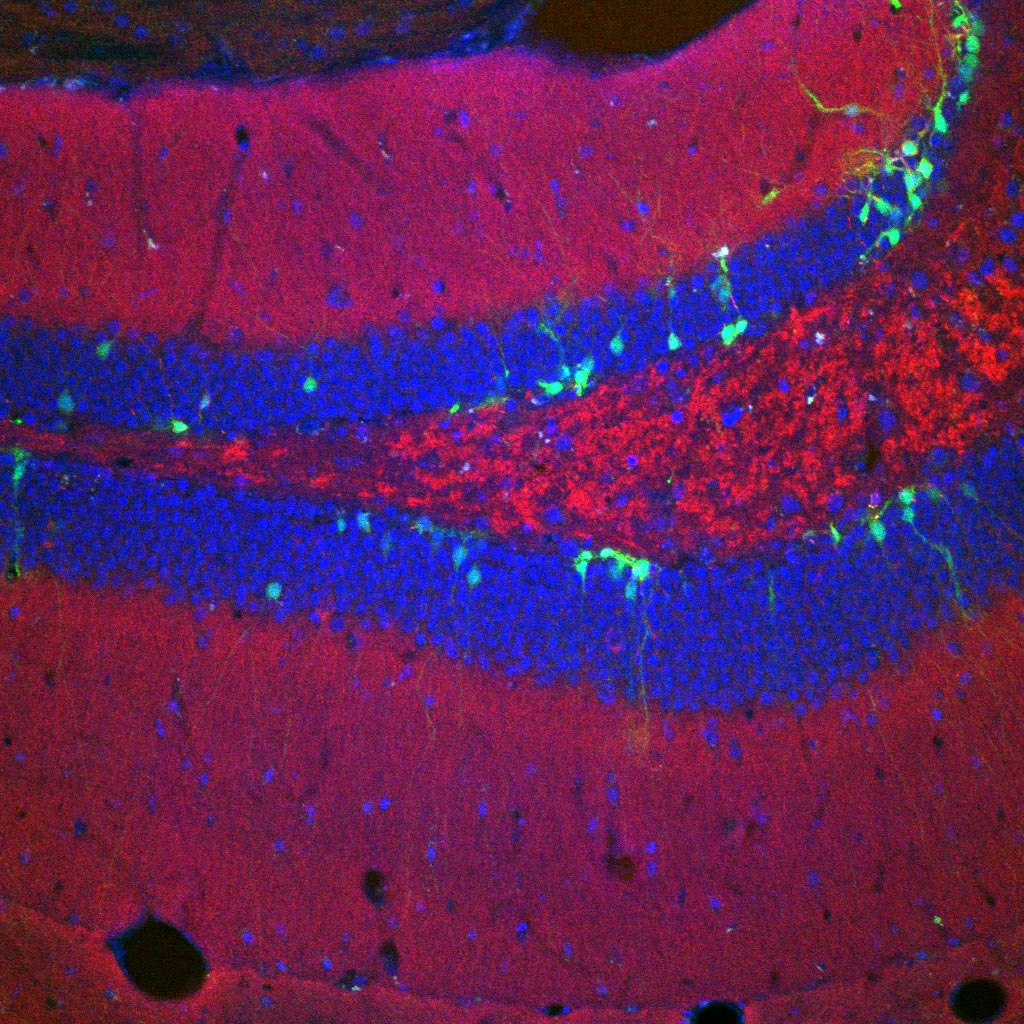
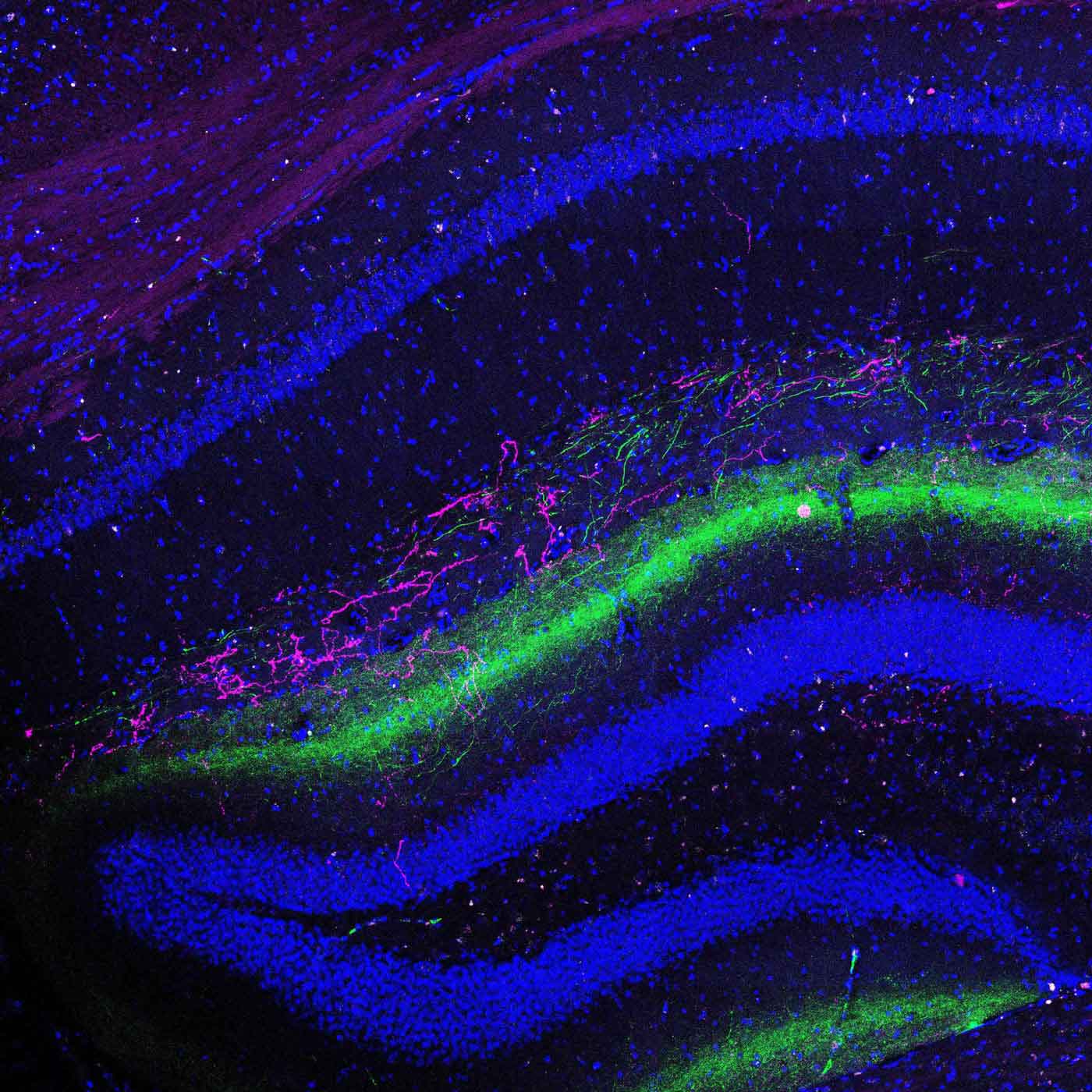
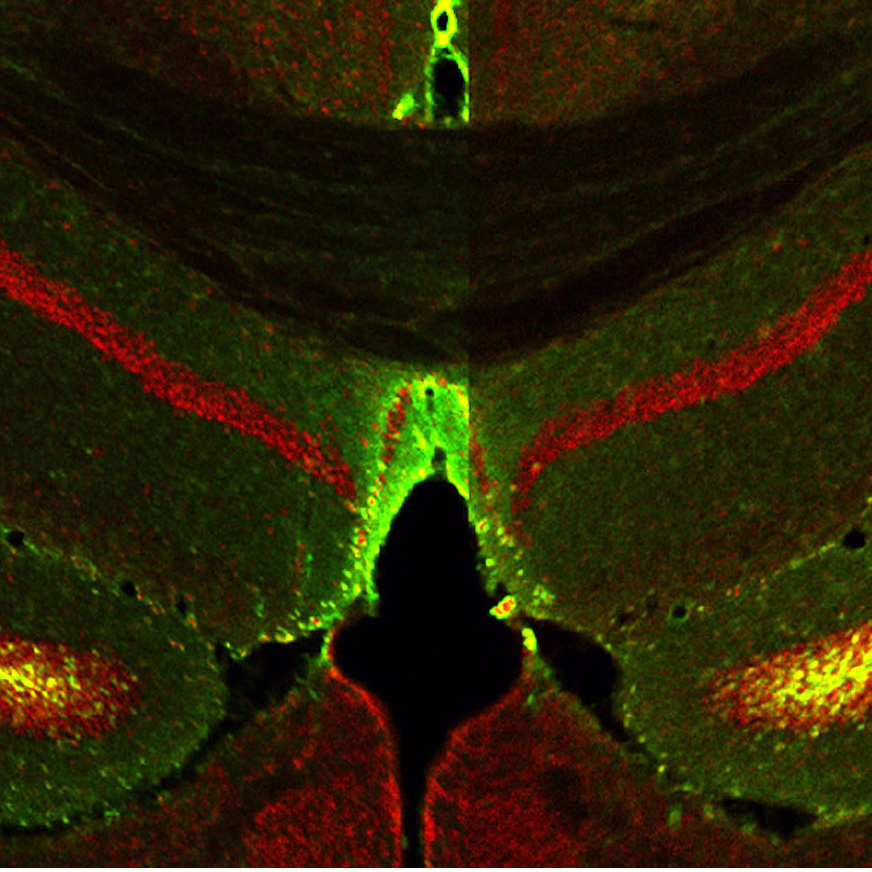
TIA1 is present in many brain regions but is particularly active in the ventral hippocampus, an area known to regulate memories associated with stress and fear. Drs. Kandel and Rayman wondered if disruptions to TIA1 function could cause disruptions in fear memory — which plays a key role in PTSD.
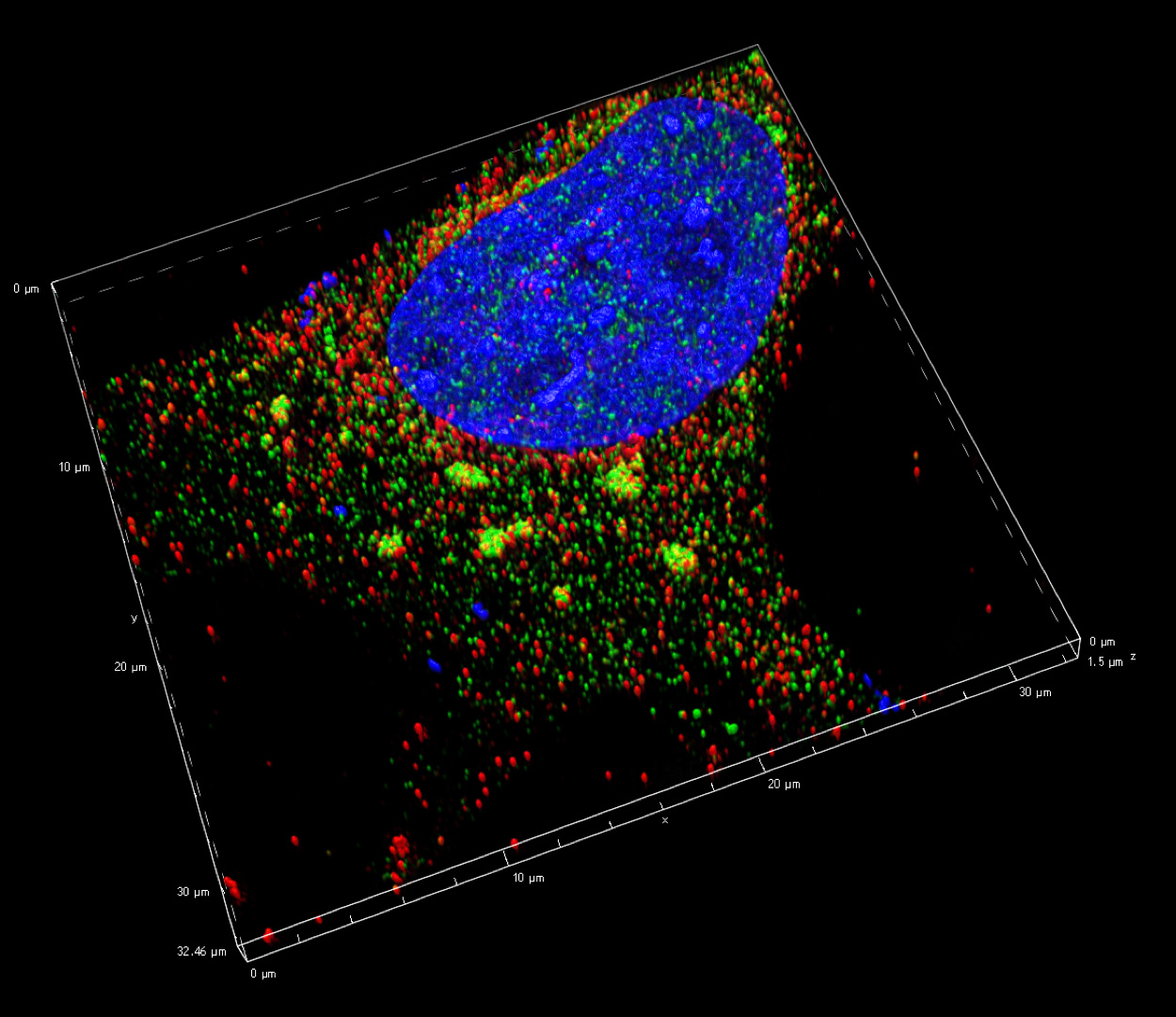
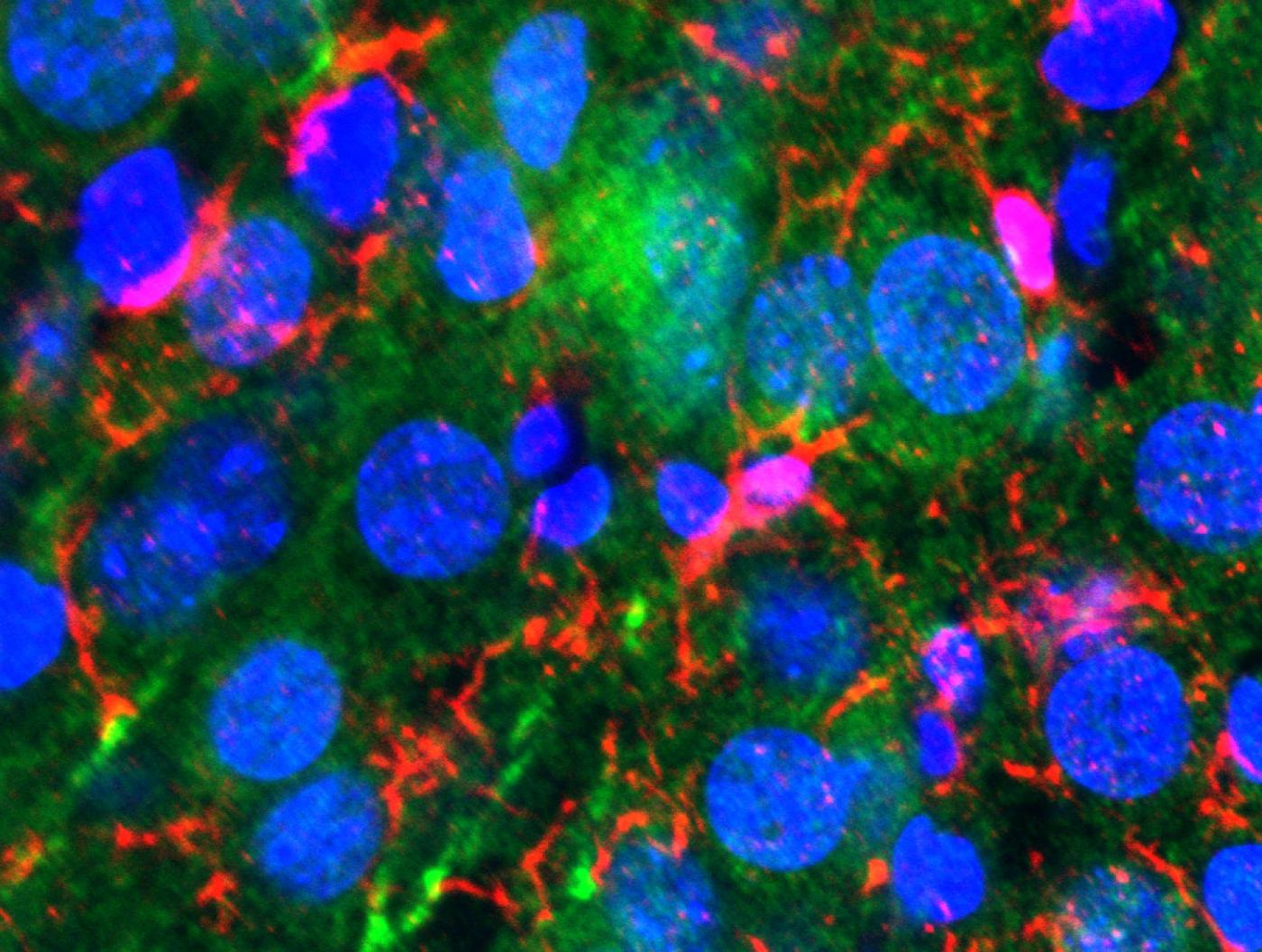
3D projection showing TIA+ stress granules in cytoplasm (relatively large yellow structures outside of nucleus (in blue) (Credit: Joseph Rayman/Kandel lab/Columbia's Zuckerman Institute).
To find out, the researchers changed the amount of TIA1 in the ventral hippocampus of both male and female mice. The scientists then trained the mice to associate an innocuous smell, that of ethanol, with a stressful experience. When placed in other ethanol-scented environments, the animals exhibited avoidance behavior: they tended to move away from the stressful scent.
But when the researchers removed TIA1, they saw a change in behavior — a change that was restricted to female mice. Removing TIA1 appeared to have no effect on the males, but the females’ avoidance behavior skyrocketed; their fearful memories were heightened significantly.
The researchers argue that this marked sex difference may be an essential key to uncovering why the prevalence of PTSD is so much higher in women, as compared to men. It also emphasizes the importance of including female mice in scientific research, a practice that has long been discouraged by the scientific community.
“The inclusion of female mice in scientific studies is a recent phenomenon; researchers once reasoned that cyclical hormone changes in females would complicate study results,” said Dr. Rayman. “But including female mice in our study proved transformational. We would never have discovered the importance of TIA1 had we not examined the female brain.”
Psychiatric disorders are notoriously complex. They are thought to be linked to many genes, each of which contributes only a small amount to the problem. But Drs. Kandel and Rayman are confident that their work can be extended to identify and treat disorders in people. For example, PTSD can ultimately be traced back to abnormal processing of fear memory. And the same brain regions responsible for fear memory in mice are responsible for fear memory in people.
“Not only that, but the gene that encodes TIA1 in mice also exists in the human genome,” said Dr. Rayman. “To search for links between TIA1 activity levels and stress responses in humans, we are currently analyzing DNA from individuals in Sweden.”
“We hope our work, combined with the work of others, will lead to the identification of a large number of genes, each of which contributes a certain amount of PTSD risk,” said Dr. Kandel, who is also a Senior Investigator at the Howard Hughes Medical Institute. “Advances in computational and theoretical modeling could soon allow us look at a person’s particular genetic composition and identify his or her risk for developing a psychiatric disorder, such as PTSD — and then offer the best way to treat it at the molecular level.”
###
This paper is titled “Genetic perturbation of TIA1 reveals a physiological role in fear memory.” Additional contributors include Joud Hijazi, Xiang Li, Nancy Kedersha and Paul Anderson.
This research was supported by the Howard Hughes Medical Institute, the Institute for Molecular Neuroscience (W81XWH-11-2-0145, Subaward 803-236), the Institute for Translational Neuroscience (W81XWH-12-2-0048, Subaward 8741sc) and Cohen Veterans Bioscience.
The authors report no financial or other conflicts of interest.