A team of Columbia University Medical Center (CUMC) researchers, led by Nobel laureate Eric R. Kandel, MD, has found that deficiency of a protein called RbAp48 in the hippocampus is a significant contributor to age-related memory loss and that this form of memory loss is reversible. The study, conducted in postmortem human brain cells and in mice, also offers the strongest causal evidence that age-related memory loss and Alzheimer’s disease are distinct conditions. The findings were published today in the online edition of Science Translational Medicine.
Our study provides compelling evidence that age-related memory loss is a syndrome in its own right, apart from Alzheimer’s.
“Our study provides compelling evidence that age-related memory loss is a syndrome in its own right, apart from Alzheimer’s. In addition to the implications for the study, diagnosis and treatment of memory disorders, these results have public health consequences,” said Dr. Kandel, who is University Professor & Kavli Professor of Brain Science, co-director of Columbia’s Mortimer B. Zuckerman Mind Brain Behavior Institute, director of the Kavli Institute for Brain Science, and senior investigator, Howard Hughes Medical Institute, at CUMC. Dr. Kandel received a share of the 2000 Nobel Prize in Physiology or Medicine for his discoveries related to the molecular basis of memory.
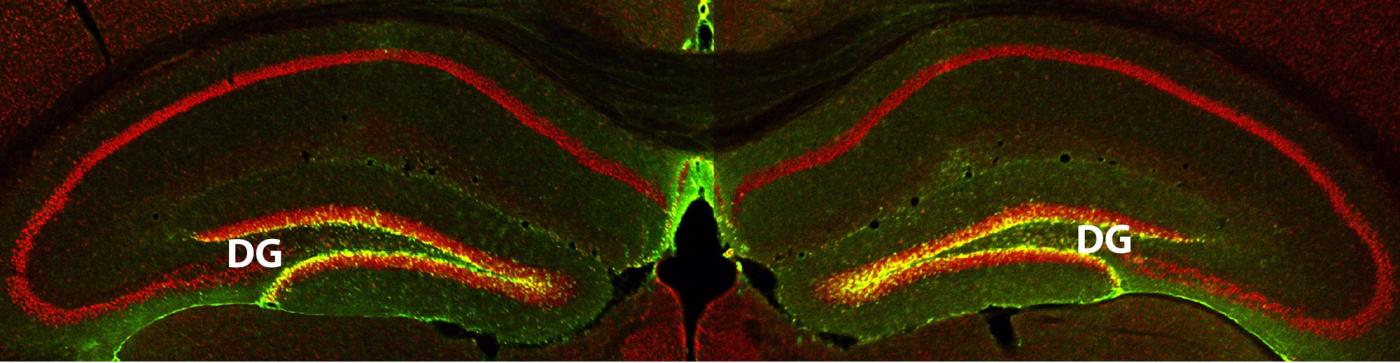
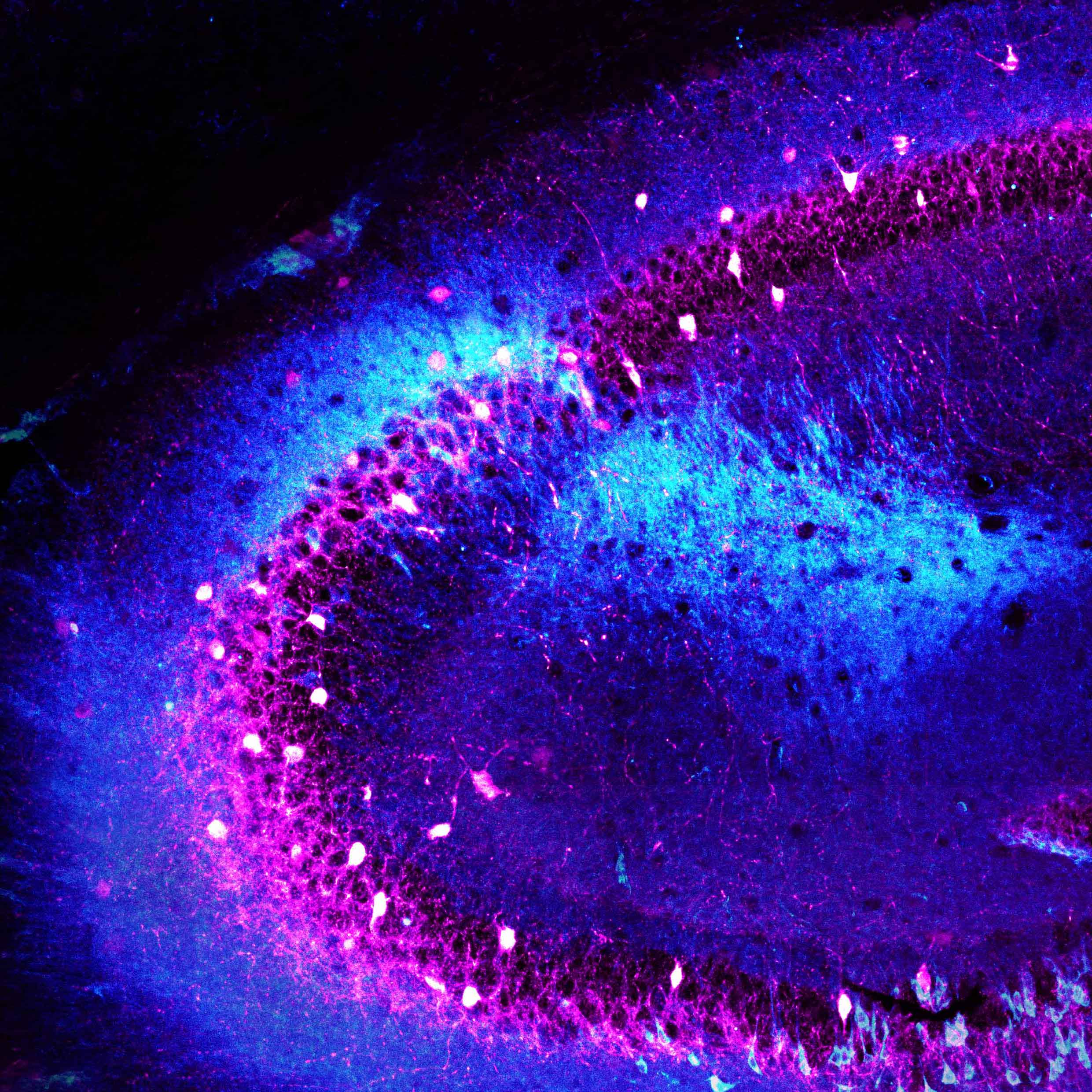
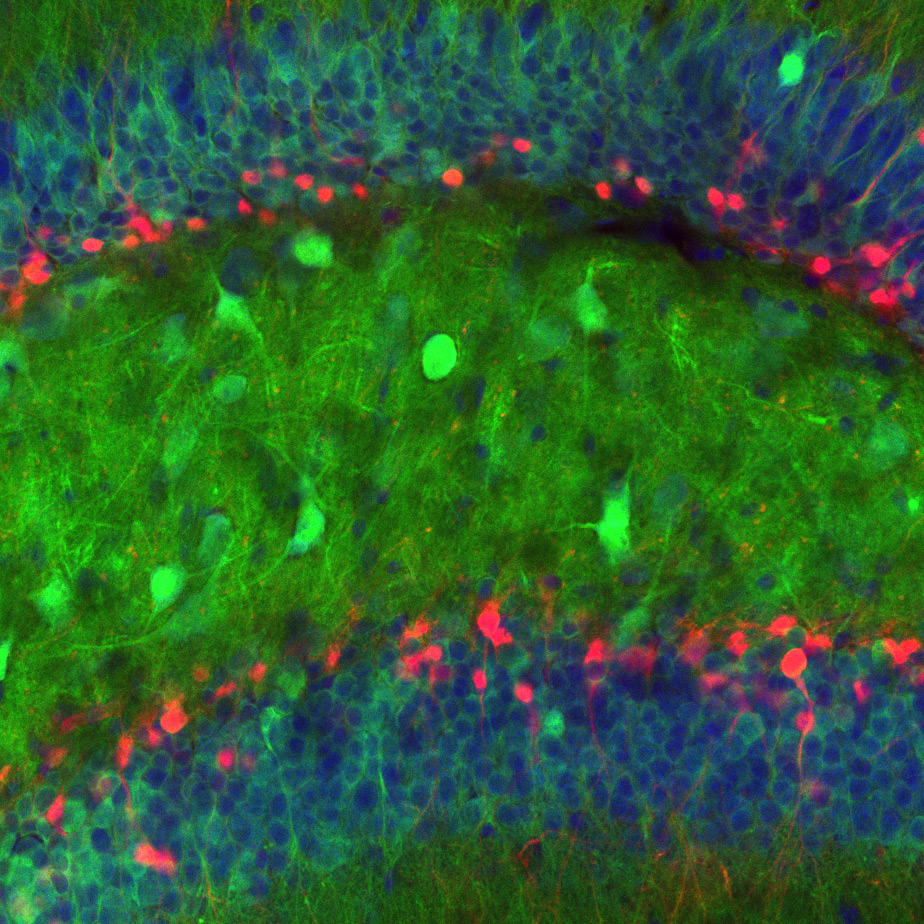
The hippocampus, a brain region that consists of several interconnected subregions, each with a distinct neuron population, plays a vital role in memory. Studies have shown that Alzheimer’s disease hampers memory by first acting on the entorhinal cortex (EC), a brain region that provides the major input pathways to the hippocampus. It was initially thought that age-related memory loss is an early manifestation of Alzheimer’s, but mounting evidence suggests that it is a distinct process that affects the dentate gyrus (DG), a subregion of the hippocampus that receives direct input from the EC.
“Until now, however, no one has been able to identify specific molecular defects involved in age-related memory loss in humans,” said co-senior author Scott A. Small, MD, the Boris and Rose Katz Professor of Neurology and director of the Alzheimer’s Research Center at CUMC.
The current study was designed to look for more direct evidence that age-related memory loss differs from Alzheimer’s disease. The researchers began by performing microarray (gene expression) analyses of postmortem brain cells from the DG of eight people, ages 33 to 88, all of whom were free of brain disease. The team also analyzed cells from their EC, which served as controls since that brain structure is unaffected by aging. The analyses identified 17 candidate genes that might be related to aging in the DG. The most significant changes occurred in a gene called RbAp48, whose expression declined steadily with aging across the study subjects.
To determine whether RbAp48 plays an active role in age-related memory loss, the researchers turned to mouse studies. “The first question was whether RbAp48 is downregulated in aged mice,” said lead author Elias Pavlopoulos, PhD, associate research scientist in neuroscience at CUMC. “And indeed, that turned out to be the case — there was a reduction of RbAp48 protein in the DG.”
When the researchers genetically inhibited RbAp48 in the brains of healthy young mice, they found the same memory loss as in aged mice, as measured by novel object recognition and water maze memory tests. When RbAp48 inhibition was turned off, the mice’s memory returned to normal.
The researchers also did functional MRI (fMRI) studies of the mice with inhibited RbAp48 and found a selective effect in the DG, similar to that seen in fMRI studies of aged mice, monkeys and humans. This effect of RbAp48 inhibition on the DG was accompanied by defects in molecular mechanisms similar to those found in aged mice. The fMRI profile and mechanistic defects of the mice with inhibited RbAp48 returned to normal when the inhibition was turned off.
In another experiment, the researchers used viral gene transfer and increased RbAp48 expression in the DG of aged mice. “We were astonished that not only did this improve the mice’s performance on the memory tests, but their performance was comparable to that of young mice,” said Dr. Pavlopoulos.
“The fact that we were able to reverse age-related memory loss in mice is very encouraging,” said Dr. Kandel. “Of course, it’s possible that other changes in the DG contribute to this form of memory loss. But at the very least, it shows that this protein is a major factor, and it speaks to the fact that age-related memory loss is due to a functional change in neurons of some sort. Unlike with Alzheimer’s, there is no significant loss of neurons.”
Finally, the study data suggest that RbAp48 protein mediates its effects, at least in part, through the PKA-CREB1-CBP pathway, which the team had found in earlier studies to be important for age-related memory loss in the mouse. According to the researchers, RbAp48 and the PKA-CREB1-CBP pathway are valid targets for therapeutic intervention. Agents that enhance this pathway have already been shown to improve age-related hippocampal dysfunction in rodents.
“Whether these compounds will work in humans is not known,” said Dr. Small. “But the broader point is that to develop effective interventions, you first have to find the right target. Now we have a good target, and with the mouse we’ve developed, we have a way to screen therapies that might be effective, be they pharmaceuticals, nutraceuticals or physical and cognitive exercises.”
“There’s been a lot of handwringing over the failures of drug trials based on findings from mouse models of Alzheimer’s,” Dr. Small said. “But this is different. Alzheimer’s does not occur naturally in the mouse. Here, we’ve caused age-related memory loss in the mouse, and we’ve shown it to be relevant to human aging."
###
The paper is titled, “A Molecular Mechanism for Age-Related Memory Loss: The Histone Binding Protein RbAp48.” The other contributors are Sidonie Jones, Stylianos Kosmidis, Maggie Close, Carla Kim, and Olga Kovalerchik, all at CUMC.
The authors declare no financial or other conflicts of interests.
The study was supported by grants from the Howard Hughes Medical Institute, the James S. McDonnell Foundation, the Broitman Foundation, the Henry M. Jackson Foundation for the Advancement of Military Medicine Inc., the McKnight Brain Research Foundation, and the National Institute on Aging (AG034618).
This news first appeared on CUMC's website.