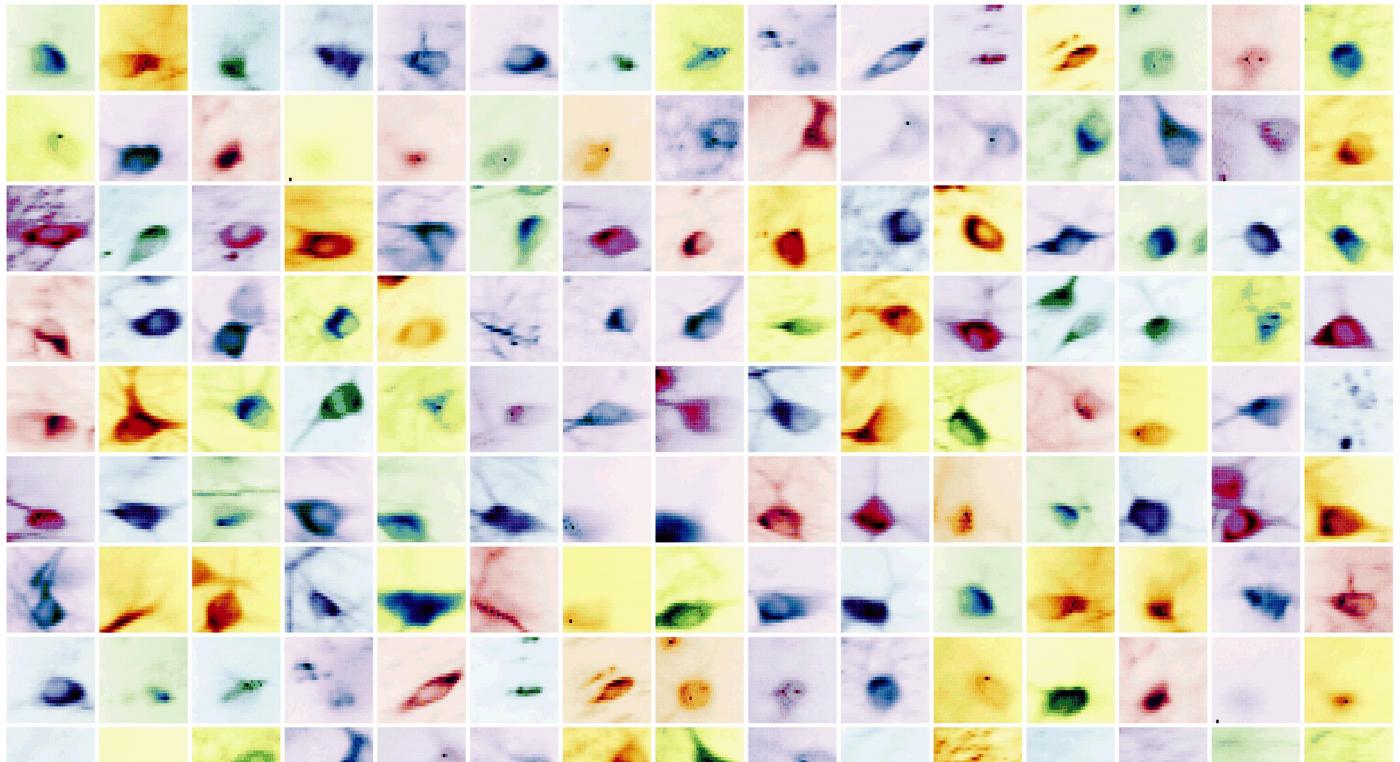
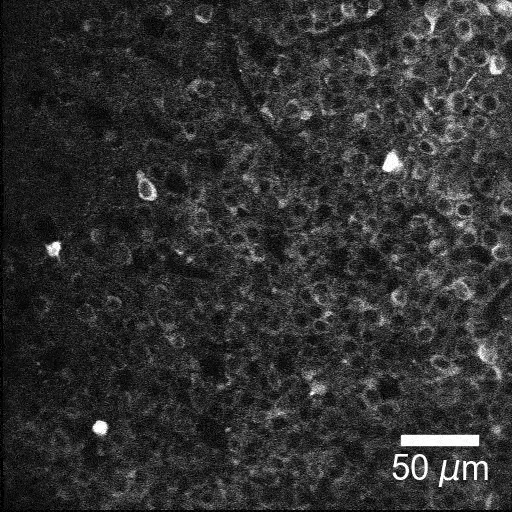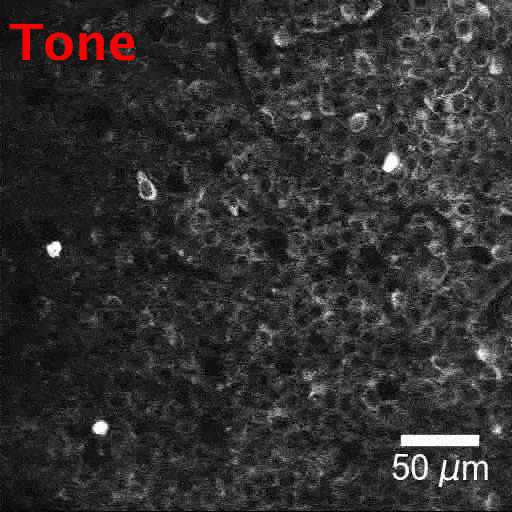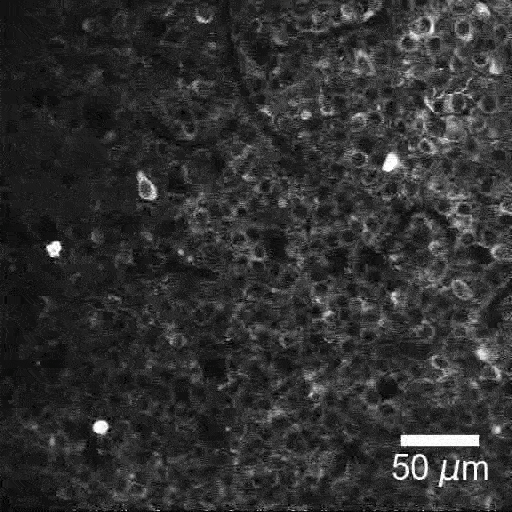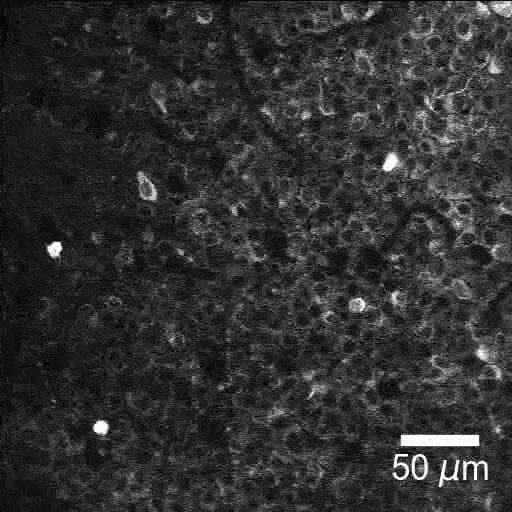
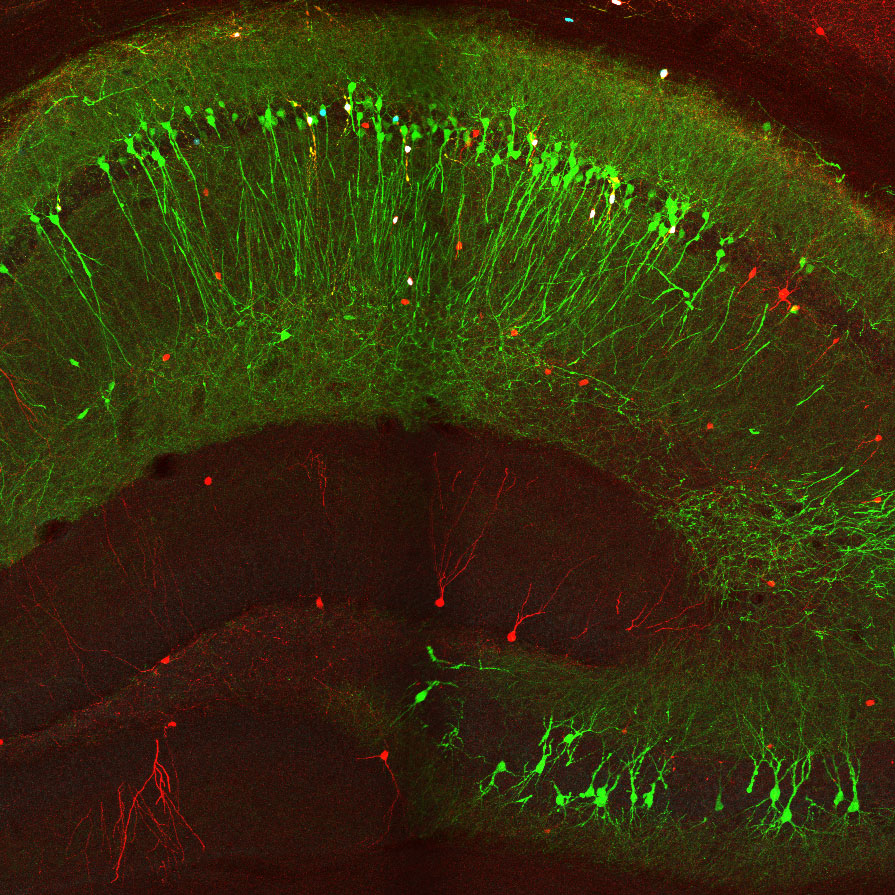
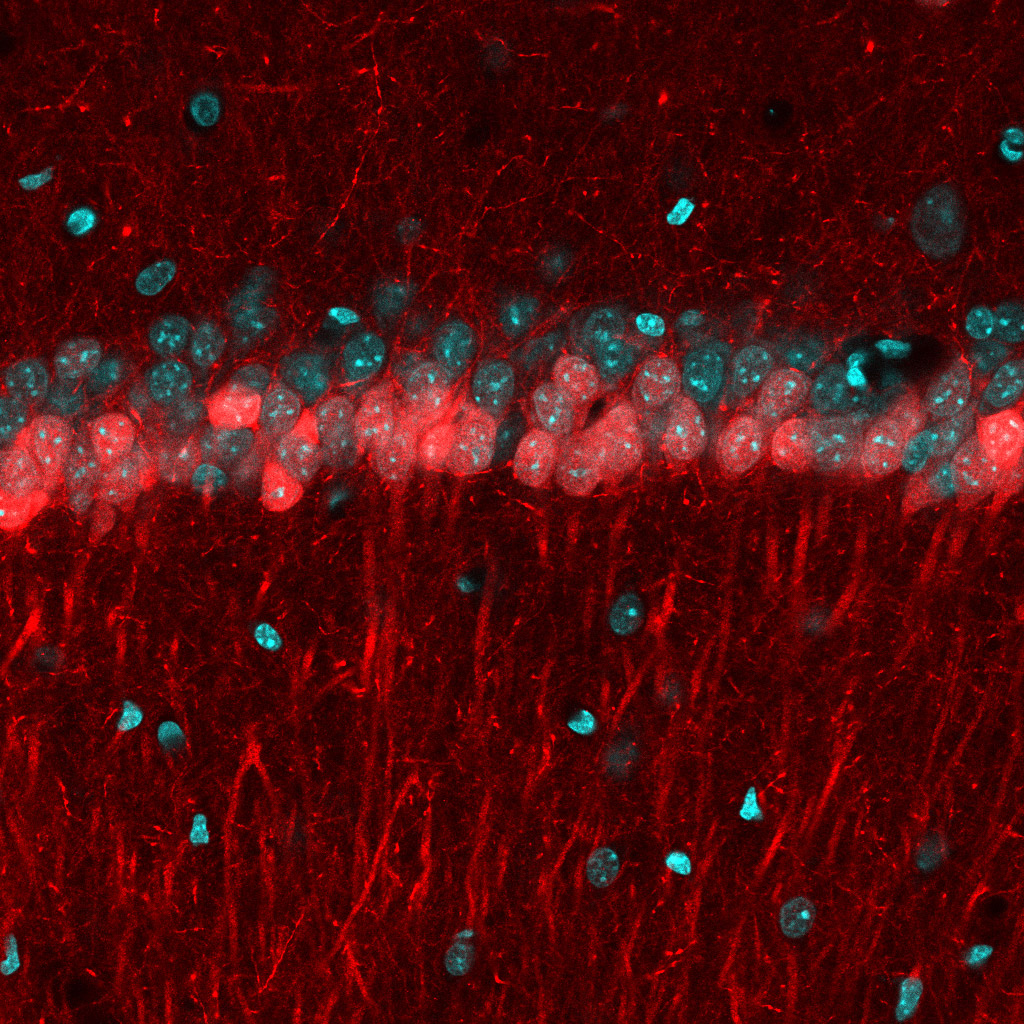
New tools and technologies are constantly pushing the boundaries of what we can see inside the brain. Pictured here are special cells that maintain a proper balance of activity across the brain. Historically difficult to find, these inhibitory neurons have now been revealed, by new research, in large numbers in mice.
We sat down with some of the neuroscientists at Columbia’s Zuckerman Institute behind the work, published October 5 in Neuron: Attila Losonczy, PhD, principal investigator at Columbia’s Zuckerman Institute and professor of neuroscience at Vagelos College of Physicians and Surgeons; and first authors Tristan Geiller, PhD, a postdoctoral researcher, and MD/PhD student Bert Vancura.
How does the brain maintain a healthy state of electrical activity, and why is this important?
Attila Losonczy: Most of the neurons that make up our brain stimulate electrical impulses in other neurons. These impulses are the informational currency that give rise to our thoughts and actions, conscious and unconscious. But without any regulation, this collective excitation can go wild. Unchecked electrical activity in the brain can cause seizures and underlie conditions such as epilepsy.
Tristan Geiller: Fortunately, the brain has special cells that keep things in balance. These inhibitory neurons put the brakes on, so that our brain doesn’t lose control. They regulate how many times other cells fire, and how often. Understanding this inhibition is key to understanding how our brains function.
How have scientists studied inhibition?
TG: Inhibitory cells in the brain are few and far between: only 5 to 10 percent of neurons. That makes them difficult to study. With the current technologies, researchers can typically investigate only a few cells at a time. On top of that, these inhibitory cells come in many flavors, with radically different shapes and functions. Most of the progress made so far has been in a brain region called the hippocampus, which is important for memory and learning. The hippocampus has been a hotbed for discovery of different types of inhibitory cells.
AL: My lab studies the hippocampus. Our goal for this project was to find a way to image lots of cells in this brain region at once. We wanted to create a comprehensive catalog of inhibitory cells, which are also called interneurons, in a part of the hippocampus called CA1, while identifying different types of these cells. There’s a jungle out there to explore, a huge variety of different interneurons.
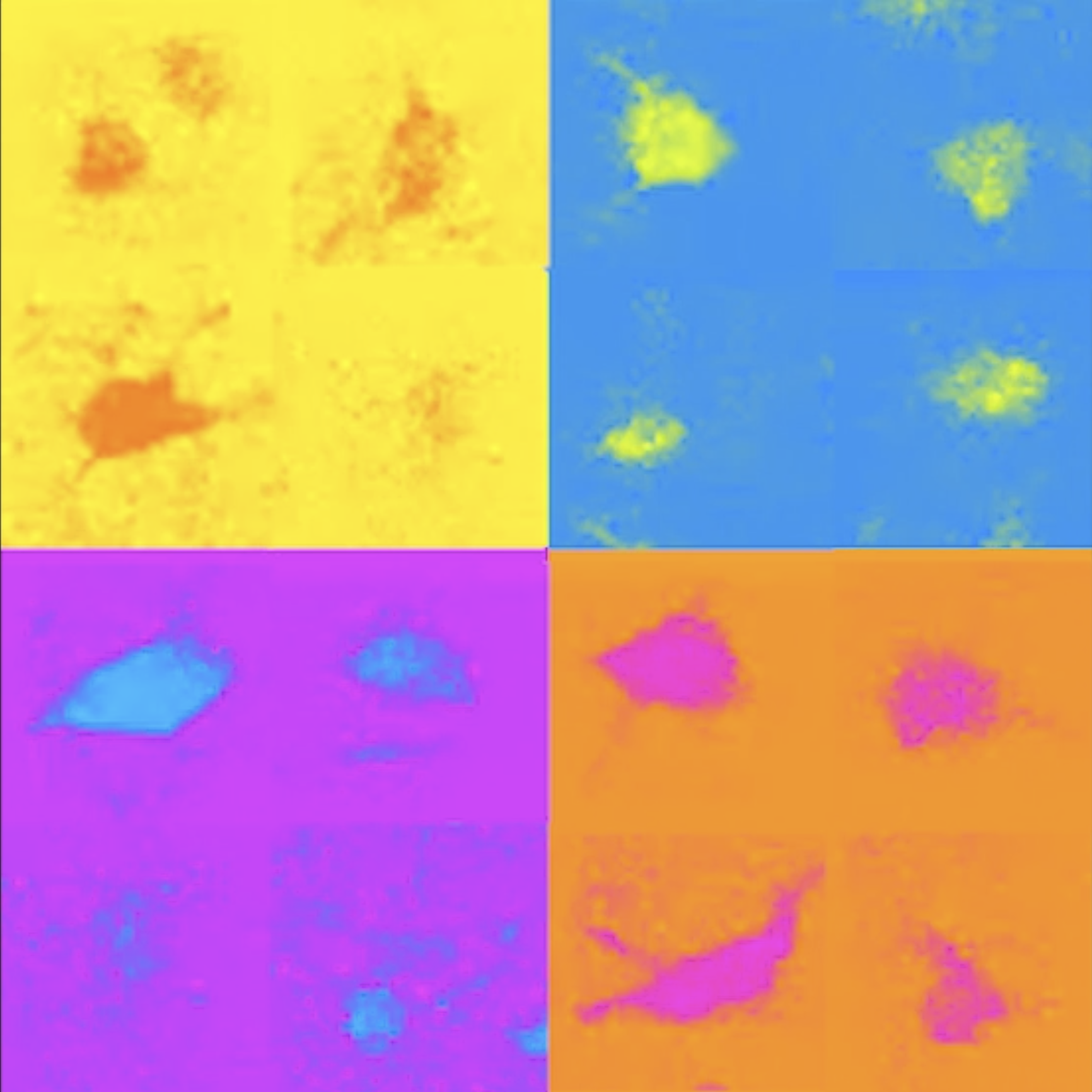
Examples of different types of inhibitory brain cells (labeled with different colors) revealed by new research (Credit: Losonczy lab / Columbia’s Zuckerman Institute)
What could this tell us about our brains?
TG: We are interested in the hippocampus because it carries out important functions. Studying the role of interneurons in its brain circuits should tell us more about how we learn and remember.
AL: Also, we think that other circuits in other parts of the brain are set up similarly to those in the hippocampus. So we believe that the principles we are learning here can be applied elsewhere. If we want to understand how collections of different cells in the brain give rise to mind and behavior, we need to investigate how the different players come together.
Bert Vancura: And here’s one big idea I find fascinating about inhibitory cells. If you look at worms, flies, mice … organisms with more complicated brain function tend to have a greater diversity of interneurons. Humans have types of interneurons never seen in animals. So maybe the key to developing advanced brain functions is gaining greater control over the brain, through inhibition.
What technological advance allowed you to visualize these cells?
BV: To look for interneurons, we took two technologies that people have used before and combined them in a new way. Using a two-photon microscope, we tracked the activity of brain cells. We could see hundreds of cells, detect their electrical impulses and map their locations in 3D. With this map in hand, we looked at tissue samples and found the same neurons we had seen with the two-photon microscope. This was a painstaking process. To locate these cells, we looked at positions of cells, shapes of the cells, how cells were connected. It was like assembling a jigsaw puzzle.
TG: Once we had located the cells, we checked for certain molecules on their surfaces. Thanks to over 30 years of work, our field has gained a lot of knowledge about which molecules are associated with which types of interneuron. We used this information to label the 3D map we had created, by type. Now we have a 3D inventory of hundreds of identified interneurons. We can use this to explore the activities of these cells and study their interactions for the first time.
What did this map show you?
AL: We noticed a few things. For one thing, we found that about 15 percent of our interneurons were spatially tuned. Now it will be fascinating to figure out how these spatially tuned interneurons interact with a class of excitatory cells, called place cells, that serve as the brain’s GPS by helping us create mental maps. The discovery of place cells, by the way, won the 2014 Nobel Prize in Physiology or Medicine.
TG: We also found evidence that interneurons can inhibit each other: the inhibition of inhibition. This is just the beginning. We’re putting all of this data online for others to use. And another group of scientists is using this data to develop a machine learning algorithm designed to automatically index and identify cells in the brain.
###
To continue exploring this research, see the new paper: “Large-scale 3D two-photon imaging of molecularly-identified CA1 interneuron dynamics in behaving mice.” Published October 5, 2020 in Neuron by Tristan Geiller, Bert Vancura, Eirini Troullinou, Spyridon Chavlis, Grigorios Tsagkatakis, Katalin Ocsai, Satoshi Terada, Panayiota Poirazi, Balazs Rozsa and Attila Losonczy.