NEW YORK, NY — The mammalian nose is a work of evolutionary art. Its millions of nerve cells, each tailored with just one of thousands of specific odor-chemical receptors encoded in the genome, can collectively distinguish a trillion distinct scents. Those sensations, in turn, inform many behaviors, from assessing food options to discerning friends from foes to sparking memories.
Today, in the journal Nature, a research team led by scientists at Columbia’s Zuckerman Institute describes a previously undetected mechanism in mice—starring the genetic molecule RNA—that could explain how each sensory cell, or neuron, in mammalian noses becomes tailored to detect a specific odor chemical.
For example, there are sensory neurons in our noses that bear receptors uniquely tuned to detect ethyl vanillin, the main odorant in vanilla, and other cells with receptors for limonene, lemon’s signature odorant.
“How sensory cells in the nose make their receptor choices has been one of the most vexing mysteries about olfaction,” said Stavros Lomvardas, PhD, a Roy and Diana Vagelos Professor and Chair of Biochemistry and Molecular Biophysics and Herbert and Florence Irving Professor of Neuroscience at Columbia's Zuckerman Institute and the Vagelos College of Physicians and Surgeons, and corresponding author on the paper. “Now, the story behind our sense of smell, or olfaction, is becoming clearer, and also more dramatic.”
The sense-refining drama he is referring to unfolds entirely within the minuscule confines of each olfactory neuron’s nucleus, where the cell’s chromosomes and genes reside. There, in a Squid Games-style, winner-takes-all competition, a developing cell’s myriad olfactory receptor genes vie with each other in a process that winnow them down, in stages, first to handful of finalists and then to a single winner. The prevailing gene is the one that determines the cell’s odorant sensitivity. In their study, Dr. Lomvardas and his team uncover details of the final stage of this process when the winner emerges from the finalist genes.
“It’s basically a battle between a 1000 contenders,” said Ariel Pourmorady, the paper’s first author and an MD-PhD candidate at the Zuckerman Institute in the Lomvardas lab.
We are reaching the edge of science fiction when it comes to the molecular and genomic details we now can observe inside a single cell’s nucleus
The action is exceedingly complex and involves a dizzying cast of molecular characters. Playing roles that either dial up or down each gene’s ability to produce olfactory receptors are a variety of gene-regulating molecules. By gathering into various alliances within the genome, these molecular players help turn specific genes on or off.
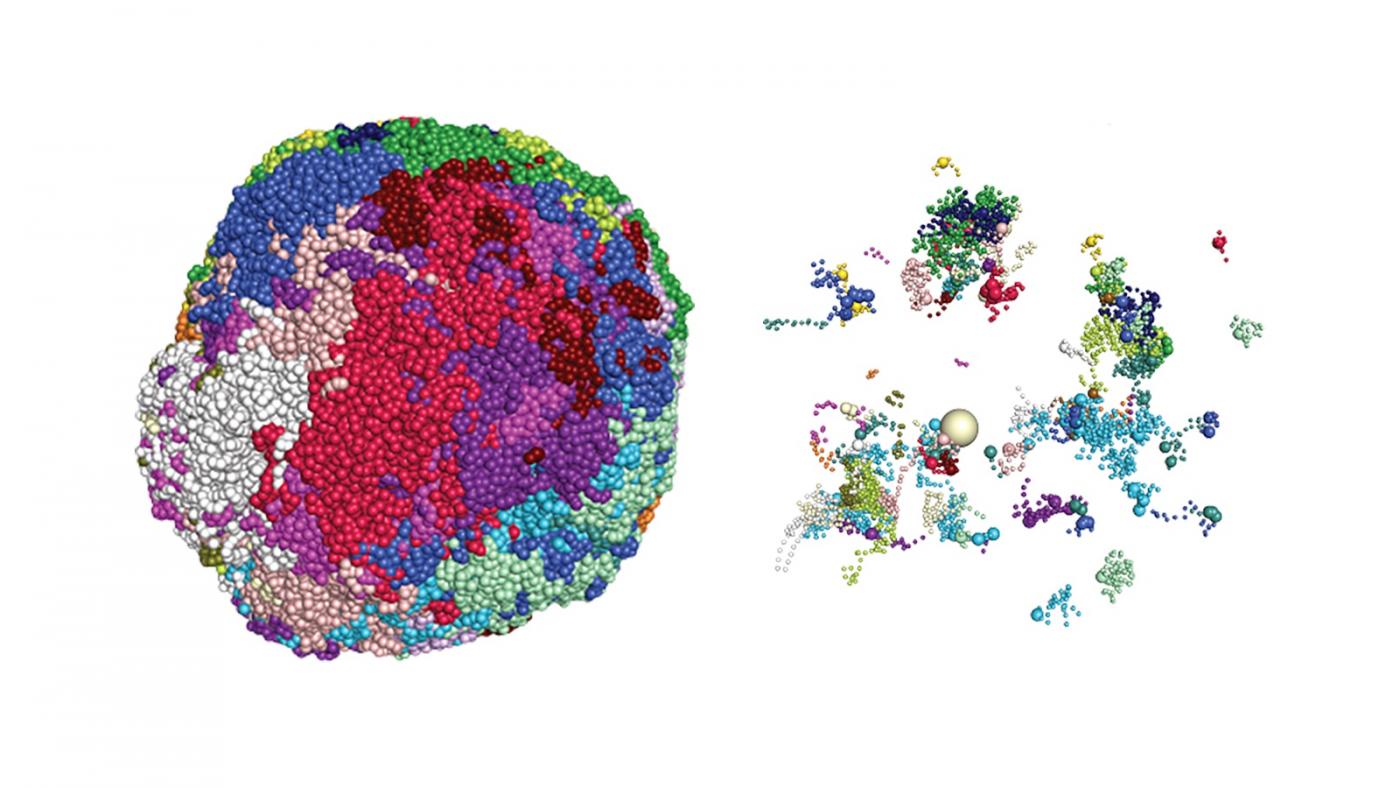
Also in the fray is another set of molecular hubs that reshape portions of the genome in ways that favor specific receptor genes. When his team first observed these in the genome in 2014, Dr. Lomvardas dubbed them “Greek Islands” because they reminded him of islands in the Aegean Sea.
“It turns out that the genome has a certain spatial organization in the nucleus and changes in this structure are pivotal when it comes to which genes are expressed into proteins, like olfactory receptors,” said Pourmorady. “We are learning just how important this process is within maturing olfactory cells.”
In their new Nature paper, the researchers summon a trove of data from mouse studies pointing toward RNA as the linchpin molecule in the olfactory system’s gene-choosing mechanism. RNA is most known as the go-between molecule that translates the genetic code embodied in DNA into protein molecules with specific cellular jobs, like detecting odorants. Using sophisticated techniques for analyzing changes in genome structure as cells mature, however, the researchers say their evidence points to a pivotal second role for the RNA.
“It looks like the RNA the cell makes during gene expression also is altering the genome’s architecture in ways that bolster the expression of one olfactory receptor gene while also shutting down all the others,” Pourmorady said.
Big gaps in this genome-controlling story remain, but the researchers say the outline
is becoming more defined. It starts with maturing olfactory cells, which initially express many receptor genes at those genomic hubs where gene-regulating molecules and complexes, including Greek Islands, converge.
Then the RNA winnows the contending olfactory-receptor genes down to one. The particular hub in each cell where the molecular stars align to produce the highest amount of RNA wins the competition. At this hub, receptor-gene expression soars. But, like a slinky saboteur, RNA from that same hub may wind its way to all the other hubs. In those locations, the RNA causes shape changes in the genome that shut down gene expression. The result is a nose’s worth of mature olfactory neurons, each of which bears on its surface only one odorant receptor.
“We are reaching the edge of science fiction when it comes to the molecular and genomic details we now can observe inside a single cell’s nucleus,” said Dr. Lomvardas. “We need to keep going back in to figure out the rest of this olfaction puzzle.”
###
The paper, "RNA-mediated symmetry breaking enables singular olfactory receptor choice," was published today in Nature.
The full list of authors includes Ariel D. Pourmorady, Elizaveta V. Bashkirova, Andrea M. Chiariello, Houda Belagzhal, Albana Kodra, Rachel Duffié, Jerome Kahiapo, Kevin Monahan, Joan Pulupa, Ira Schieren, Alexa Osterhoudt, Job Dekker, Mario Nicodemi and Stavros Lomvardas.
The authors declare no competing interests.